Central Neural Circuits Orchestrating Thermogenesis, Sleep-Wakefulness States and General Anesthesia States
- PMID: 33632102
- PMCID: PMC9199556
- DOI: 10.2174/1570159X19666210225152728
Central Neural Circuits Orchestrating Thermogenesis, Sleep-Wakefulness States and General Anesthesia States
Abstract
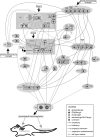
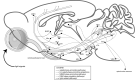
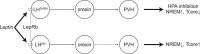
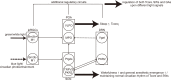
Great progress has been made in specifically identifying the central neural circuits (CNCs) of the core body temperature (Tcore), sleep-wakefulness states (SWs), and general anesthesia states (GAs), mainly utilizing optogenetic or chemogenetic manipulations. We summarize the neuronal populations and neural pathways of these three CNCs, which gives evidence for the orchestration within these three CNCs, and the integrative regulation of these three CNCs by different environmental light signals. We also outline some transient receptor potential (TRP) channels that function in the CNCs-Tcore and are modulated by some general anesthetics, which makes TRP channels possible targets for addressing the general-anestheticsinduced- hypothermia (GAIH). We suggest this review will provide new orientations for further consummating these CNCs and elucidating the central mechanisms of GAIH.
Keywords: Central neural circuits; TRP channels; body temperature regulation; chemogenetics; general anesthesia; intrinsically photosensitive retinal ganglion cells; optogenetics; sleep-wakefulness states.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures



Similar articles
-
Recent advances in neural mechanism of general anesthesia induced unconsciousness: insights from optogenetics and chemogenetics.Front Pharmacol. 2024 Apr 9;15:1360864. doi: 10.3389/fphar.2024.1360864. eCollection 2024. Front Pharmacol. 2024. PMID: 38655183 Free PMC article. Review.
-
Elucidation of Neural Circuits Involved in the Regulation of Sleep/Wakefulness Using Optogenetics.Adv Exp Med Biol. 2021;1293:391-406. doi: 10.1007/978-981-15-8763-4_25. Adv Exp Med Biol. 2021. PMID: 33398828
-
Neural Substrates for the Regulation of Sleep and General Anesthesia.Curr Neuropharmacol. 2022;20(1):72-84. doi: 10.2174/1570159X19666211214144639. Curr Neuropharmacol. 2022. PMID: 34906058 Free PMC article. Review.
-
Optogenetic Manipulation of Neural Circuits During Monitoring Sleep/wakefulness States in Mice.J Vis Exp. 2019 Jun 19;(148). doi: 10.3791/58613. J Vis Exp. 2019. PMID: 31282883
-
GABAergic Neurons in the Dorsal-Intermediate Lateral Septum Regulate Sleep-Wakefulness and Anesthesia in Mice.Anesthesiology. 2021 Sep 1;135(3):463-481. doi: 10.1097/ALN.0000000000003868. Anesthesiology. 2021. PMID: 34259824
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

