Ectopic gut colonization: a metagenomic study of the oral and gut microbiome in Crohn's disease
- PMID: 33632307
- PMCID: PMC7905567
- DOI: 10.1186/s13099-021-00409-5
Ectopic gut colonization: a metagenomic study of the oral and gut microbiome in Crohn's disease
Abstract
Background: This study aims to characterize, the gut and oral microbiome in Asian subjects with Crohn's disease (CD) using whole genome shotgun sequencing, thereby allowing for strain-level comparison.
Methods: A case-control study with age, sex and ethnicity matched healthy controls was conducted. CD subjects were limited to well-controlled patients without oral manifestations. Fecal and saliva samples were collected for characterization of gut and oral microbiome respectively. Microbial DNA were extracted, libraries prepared and sequenced reads profiled. Taxonomic diversity, taxonomic association, strain typing and microbial gene pathway analyses were conducted.
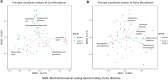
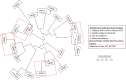
Results: The study recruited 25 subjects with CD and 25 healthy controls. The oral microbe Streptococcus salivarius was found to be enriched and of concordant strains in the gut and oral microbiome of Crohn's disease subjects. This was more likely in CD subjects with higher Crohn's Disease Activity Index (184.3 ± 2.9 vs 67.1 ± 82.5, p = 0.012) and active disease status (Diarrhoea/abdominal pain/blood-in-stool/fever and fatigue) (p = 0.016). Gut species found to be significantly depleted in CD compared to control (Relative abundance: Median[Range]) include: Faecalibacterium prausnitzii (0.03[0.00-4.56] vs 13.69[5.32-18.71], p = 0.010), Roseburia inulinivorans (0.00[0.00-0.03] vs 0.21[0.01-0.53], p = 0.010) and Alistipes senegalensis (0.00[0.00-0.00] vs 0.00[0.00-0.02], p = 0.029). While Clostridium nexile (0.00[0.00-0.12] vs 0.00[0.00-0.00], p = 0.038) and Ruminococcus gnavus (0.43[0.02-0.33] vs 0.00[0.00-0.13], p = 0.043) were found to be enriched. C. nexile enrichment was not found in CD subjects of European descent. Microbial arginine (Linear-discriminant-analysis: 3.162, p = 0.001) and isoprene (Linear-discriminant-analysis: 3.058, p < 0.001) pathways were found at a higher relative abundance level in gut microbiome of Crohn's disease.
Conclusions: There was evidence of ectopic gut colonization by oral bacteria, especially during the active phase of CD. Previously studied gut microbial differences were detected, in addition to novel associations which could have resulted from geographical/ethnic differences to subjects of European descent. Differences in microbial pathways provide possible targets for microbiome modification.
Keywords: Crohn’s disease; Gastrointestinal microbiome; Metagenomics; Oral microbiome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The Fecal Microbiome in Quiescent Crohn's Disease with Persistent Gastrointestinal Symptoms Show Enrichment of Oral Microbes But Depletion of Butyrate and Indole Producers.medRxiv [Preprint]. 2023 May 17:2023.05.16.23290065. doi: 10.1101/2023.05.16.23290065. medRxiv. 2023. PMID: 37292648 Free PMC article. Preprint.
-
Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease.Digestion. 2016;93(1):59-65. doi: 10.1159/000441768. Epub 2016 Jan 14. Digestion. 2016. PMID: 26789999
-
Oral Microbiome of Crohn's Disease Patients With and Without Oral Manifestations.J Crohns Colitis. 2022 Nov 1;16(10):1628-1636. doi: 10.1093/ecco-jcc/jjac063. J Crohns Colitis. 2022. PMID: 35511486 Free PMC article.
-
The Microbiome in Crohn's Disease: Role in Pathogenesis and Role of Microbiome Replacement Therapies.Gastroenterol Clin North Am. 2017 Sep;46(3):481-492. doi: 10.1016/j.gtc.2017.05.004. Epub 2017 Jul 19. Gastroenterol Clin North Am. 2017. PMID: 28838410 Review.
-
Contribution of the Gut Microbiome to the Perpetuation of Inflammation in Crohn's Disease: A Systematic Review.Cureus. 2024 Aug 24;16(8):e67672. doi: 10.7759/cureus.67672. eCollection 2024 Aug. Cureus. 2024. PMID: 39314611 Free PMC article. Review.
Cited by
-
The oral-gut axis: Salivary and fecal microbiome dysbiosis in patients with inflammatory bowel disease.Front Cell Infect Microbiol. 2022 Oct 7;12:1010853. doi: 10.3389/fcimb.2022.1010853. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275026 Free PMC article.
-
The oral-gut axis: a missing piece in the IBD puzzle.Inflamm Regen. 2023 Nov 6;43(1):54. doi: 10.1186/s41232-023-00304-3. Inflamm Regen. 2023. PMID: 37932859 Free PMC article. Review.
-
OSaMPle workflow for salivary metaproteomics analysis reveals dysbiosis in inflammatory bowel disease patients.NPJ Biofilms Microbiomes. 2025 Apr 23;11(1):63. doi: 10.1038/s41522-025-00692-z. NPJ Biofilms Microbiomes. 2025. PMID: 40268913 Free PMC article.
-
In Vitro Modelling of Oral Microbial Invasion in the Human Colon.Microbiol Spectr. 2023 Mar 27;11(2):e0434422. doi: 10.1128/spectrum.04344-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36971547 Free PMC article.
-
Smoking-induced microbial dysbiosis in health and disease.Clin Sci (Lond). 2022 Sep 30;136(18):1371-1387. doi: 10.1042/CS20220175. Clin Sci (Lond). 2022. PMID: 36156126 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

