Intestinal organoids in farm animals
- PMID: 33632315
- PMCID: PMC7905770
- DOI: 10.1186/s13567-021-00909-x
Intestinal organoids in farm animals
Abstract
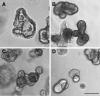
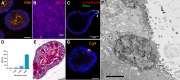
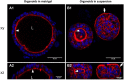
In livestock species, the monolayer of epithelial cells covering the digestive mucosa plays an essential role for nutrition and gut barrier function. However, research on farm animal intestinal epithelium has been hampered by the lack of appropriate in vitro models. Over the past decade, methods to culture livestock intestinal organoids have been developed in pig, bovine, rabbit, horse, sheep and chicken. Gut organoids from farm animals are obtained by seeding tissue-derived intestinal epithelial stem cells in a 3-dimensional culture environment reproducing in vitro the stem cell niche. These organoids can be generated rapidly within days and are formed by a monolayer of polarized epithelial cells containing the diverse differentiated epithelial progeny, recapitulating the original structure and function of the native epithelium. The phenotype of intestinal organoids is stable in long-term culture and reflects characteristics of the digestive segment of origin. Farm animal intestinal organoids can be amplified in vitro, cryopreserved and used for multiple experiments, allowing an efficient reduction of the use of live animals for experimentation. Most of the studies using livestock intestinal organoids were used to investigate host-microbe interactions at the epithelial surface, mainly focused on enteric infections with viruses, bacteria or parasites. Numerous other applications of farm animal intestinal organoids include studies on nutrient absorption, genome editing and bioactive compounds screening relevant for agricultural, veterinary and biomedical sciences. Further improvements of the methods used to culture intestinal organoids from farm animals are required to replicate more closely the intestinal tissue complexity, including the presence of non-epithelial cell types and of the gut microbiota. Harmonization of the methods used to culture livestock intestinal organoids will also be required to increase the reproducibility of the results obtained in these models. In this review, we summarize the methods used to generate and cryopreserve intestinal organoids in farm animals, present their phenotypes and discuss current and future applications of this innovative culture system of the digestive epithelium.
Keywords: Bovine; Chicken; Culture; Enteroids; Epithelium; Gut; Horse; Monolayer; Pig; Polarity; Rabbit.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Vergauwen H (2015) The IPEC-J2 Cell Line. In: Verhoeckx K, Cotter P, Lopez-Exposito I et al. (eds) The Impact of Food Bioactives on Health: in vitro and ex vivo models. Cham (CH), pp 125–134. doi:10.1007/978-3-319-16104-4_12 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

