Chronic obstructive pulmonary disease in severe mental illness: A timely diagnosis to advance the process of quitting smoking
- PMID: 33632347
- PMCID: PMC8057420
- DOI: 10.1192/j.eurpsy.2021.12
Chronic obstructive pulmonary disease in severe mental illness: A timely diagnosis to advance the process of quitting smoking
Abstract
Background: This study has two main objectives: to describe the prevalence of undetected chronic obstructive pulmonary disease (COPD) in a clinical sample of smokers with severe mental illness (SMI), and to assess the value of the Tobacco Intensive Motivational Estimated Risk tool, which informs smokers of their respiratory risk and uses brief text messages to reinforce intervention.
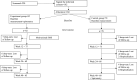
Method: A multicenter, randomized, open-label, and active-controlled clinical trial, with a 12-month follow-up. Outpatients with schizophrenia (SZ) and bipolar disorder were randomized either to the experimental group-studied by spirometry and informed of their calculated lung age and degree of obstruction (if any)-or to the active control group, who followed the 5 A's intervention.
Results: The study sample consisted of 160 patients (71.9% SZ), 78.1% of whom completed the 12-month follow-up. Of the patients who completed the spirometry test, 23.9% showed evidence of COPD (77.8% in moderate or severe stages). TIMER was associated with a significant reduction in tobacco use at week 12 and in the long term, 21.9% of patients reduced consumption and 14.6% at least halved it. At week 48, six patients (7.3%) allocated to the experimental group achieved the seven-day smoking abstinence confirmed by CO (primary outcome in terms of efficacy), compared to three (3.8%) in the control group.
Conclusion: In this clinical pilot trial, one in four outpatients with an SMI who smoked had undiagnosed COPD. An intensive intervention tool favors the early detection of COPD and maintains its efficacy to quit smoking, compared with the standard 5 A's intervention.
Keywords: COPD; bipolar disorder; early detection; schizophrenia; smoking.
Conflict of interest statement
Vicent Balanzá-Martínez has received grants and served as consultant, advisor, or continuing medical education (CME) speaker during the last 5 years for the following entities: Angelini Spain, Angelini Portugal, Bristol-Myers-Squibb, Ferrer, Janssen, Juste, Lundbeck, Nutrición Médica, and Otsuka. He acknowledges the national grant PI16/01770 from the Instituto de Salud Carlos III, ISCIII (The PROBILIFE study).
Luis Gutiérrez-Rojas has been speaker for and advisory board member of Bristol-Myers Squibb, Janssen-Cilag, Astra-Zeneca, Rovi, Lundbeck, Otsuka, GSK, and Pfizer.
José Ángel Alcalá during the last 5 years, has received grants and served as
consultant, advisor, or CME speaker for the following entities: Adamed,
Angelini, Casen Recordati, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, and Servier.
Julio Bobes has received research grants and served as consultant, advisor, or speaker within de last 5 years for: AB-Biotics, Acadia Pharmaceuticals, Alkermes, Allergan, Ambrosseti-Angelini, Biogen, Casen Recordati, D&A Pharma, Exeltis, Gilead, Indivior, GW Pharmaceuticals, Janssen-Cilag, Jazz Pharmaceuticals, Lundbeck, Mundipharma, Newron, Otsuka, Pfizer, Roche, Sage Therapeutics, Servier, Schwabe Farma Ibérica, Shire, Takeda, research funding from the Spanish Ministry of Economy and Competitiveness—Centro de Investigación Biomedica en Red area de Salud Mental (CIBERSAM) and Instituto de Salud Carlos III-, Spanish Ministry of Health, Social Services and Equality—Plan Nacional sobre Drogas- and the 7th Framework Program of the European Union.
All other researchers report no biomedical financial interests or potential conflicts of interests.
Figures
Similar articles
-
Smoking cessation opportunities in severe mental illness (tobacco intensive motivational and estimate risk - TIMER-): study protocol for a randomized controlled trial.Trials. 2019 Jan 14;20(1):47. doi: 10.1186/s13063-018-3139-9. Trials. 2019. PMID: 30642365 Free PMC article.
-
Effectiveness of spirometry as a motivational tool for smoking cessation: a clinical trial, the ESPIMOAT study.BMC Fam Pract. 2013 Dec 5;14:185. doi: 10.1186/1471-2296-14-185. BMC Fam Pract. 2013. PMID: 24308728 Free PMC article. Clinical Trial.
-
[Early detection of COPD in smoking cessation outpatients].Tunis Med. 2015 Jul;93(7):458-64. Tunis Med. 2015. PMID: 26757504 French.
-
Smoking cessation treatment for COPD smokers: the role of counselling.Monaldi Arch Chest Dis. 2013 Mar;79(1):33-7. doi: 10.4081/monaldi.2013.107. Monaldi Arch Chest Dis. 2013. PMID: 23741944 Review.
-
[Smoking cessation in smokers with chronic obstructive pulmonary disease].Rev Mal Respir. 2014 Dec;31(10):937-60. doi: 10.1016/j.rmr.2014.07.001. Epub 2014 Aug 18. Rev Mal Respir. 2014. PMID: 25496790 Review. French.
Cited by
-
Low lung function in Bipolar Disorder and Schizophrenia: a hidden risk.Front Physiol. 2024 Apr 25;15:1335798. doi: 10.3389/fphys.2024.1335798. eCollection 2024. Front Physiol. 2024. PMID: 38737830 Free PMC article.
-
Cigarette smoking and risk of suicide in bipolar disorder: a systematic review.Front Psychiatry. 2023 May 19;14:1179733. doi: 10.3389/fpsyt.2023.1179733. eCollection 2023. Front Psychiatry. 2023. PMID: 37275988 Free PMC article. Review.
-
How to Enhance the Diagnosis of Early Stages of Chronic Obstructive Pulmonary Disease (COPD)? The Role of Mobile Spirometry in COPD Screening and Diagnosis-A Systematic Review.Adv Respir Med. 2024 Mar 27;92(2):158-174. doi: 10.3390/arm92020018. Adv Respir Med. 2024. PMID: 38666812 Free PMC article.
-
Respiratory disease in people with bipolar disorder: a systematic review and meta-analysis.Mol Psychiatry. 2025 Feb;30(2):777-785. doi: 10.1038/s41380-024-02793-1. Epub 2024 Nov 14. Mol Psychiatry. 2025. PMID: 39543369
-
Inflammatory Response in SARS-CoV-2 Infection of Patients with Schizophrenia and Long-Term Antipsychotic Treatment.Neuropsychiatr Dis Treat. 2021 Oct 2;17:3053-3060. doi: 10.2147/NDT.S325062. eCollection 2021. Neuropsychiatr Dis Treat. 2021. PMID: 34629871 Free PMC article.
References
-
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen DAN, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical