Cardiac magnetic resonance fingerprinting: Trends in technical development and potential clinical applications
- PMID: 33632415
- PMCID: PMC8366914
- DOI: 10.1016/j.pnmrs.2020.10.001
Cardiac magnetic resonance fingerprinting: Trends in technical development and potential clinical applications
Abstract
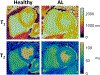
Quantitative cardiac magnetic resonance has emerged in recent years as an approach for evaluating a range of cardiovascular conditions, with T1 and T2 mapping at the forefront of these developments. Cardiac Magnetic Resonance Fingerprinting (cMRF) provides a rapid and robust framework for simultaneous quantification of myocardial T1 and T2 in addition to other tissue properties. Since the advent of cMRF, a number of technical developments and clinical validation studies have been reported. This review provides an overview of cMRF, recent technical developments, healthy subject and patient studies, anticipated technical improvements, and potential clinical applications. Recent technical developments include slice profile and pulse efficiency corrections, improvements in image reconstruction, simultaneous multislice imaging, 3D whole-ventricle imaging, motion-resolved imaging, fat-water separation, and machine learning for rapid dictionary generation. Future technical developments in cMRF, such as B0 and B1 field mapping, acceleration of acquisition and reconstruction, imaging of patients with implanted devices, and quantification of additional tissue properties are also described. Potential clinical applications include characterization of infiltrative, inflammatory, and ischemic cardiomyopathies, tissue characterization in the left atrium and right ventricle, post-cardiac transplantation assessment, reduction of contrast material, pre-procedural planning for electrophysiology interventions, and imaging of patients with implanted devices.
Keywords: Cardiac MRI; Magnetic Resonance Fingerprinting; Multiparametric MRI; Quantitative MRI; Relaxometry.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following institutional agreements that could appear to influence the work reported in this paper. The Cleveland Clinic has research agreements with Siemens Healthineers and Philips Healthcare. King’s College London has research agreements with Siemens Healthineers and Philips Healthcare. The University of Michigan has a research agreement with Siemens Healthineers.
Figures

Similar articles
-
Cardiac Magnetic Resonance Fingerprinting: Potential Clinical Applications.Curr Cardiol Rep. 2023 Mar;25(3):119-131. doi: 10.1007/s11886-022-01836-9. Epub 2023 Feb 18. Curr Cardiol Rep. 2023. PMID: 36805913 Free PMC article. Review.
-
Myocardial T1 and T2 quantification and water-fat separation using cardiac MR fingerprinting with rosette trajectories at 3T and 1.5T.Magn Reson Med. 2021 Jan;85(1):103-119. doi: 10.1002/mrm.28404. Epub 2020 Jul 27. Magn Reson Med. 2021. PMID: 32720408 Free PMC article.
-
Simultaneous multislice cardiac magnetic resonance fingerprinting using low rank reconstruction.NMR Biomed. 2019 Feb;32(2):e4041. doi: 10.1002/nbm.4041. Epub 2018 Dec 18. NMR Biomed. 2019. PMID: 30561779 Free PMC article.
-
Water-fat Dixon cardiac magnetic resonance fingerprinting.Magn Reson Med. 2020 Jun;83(6):2107-2123. doi: 10.1002/mrm.28070. Epub 2019 Nov 18. Magn Reson Med. 2020. PMID: 31736146 Free PMC article.
-
Cardiac Magnetic Resonance Fingerprinting: Technical Developments and Initial Clinical Validation.Curr Cardiol Rep. 2019 Jul 27;21(9):91. doi: 10.1007/s11886-019-1181-1. Curr Cardiol Rep. 2019. PMID: 31352620 Free PMC article. Review.
Cited by
-
Current Applications and Future Development of Magnetic Resonance Fingerprinting in Diagnosis, Characterization, and Response Monitoring in Cancer.Cancers (Basel). 2021 Sep 22;13(19):4742. doi: 10.3390/cancers13194742. Cancers (Basel). 2021. PMID: 34638229 Free PMC article. Review.
-
Recent Progress of Cardiac MRI for Nuclear Medicine Professionals.Nucl Med Mol Imaging. 2024 Dec;58(7):431-448. doi: 10.1007/s13139-024-00850-9. Epub 2024 Feb 14. Nucl Med Mol Imaging. 2024. PMID: 39635630 Review.
-
Emerging Trends in Magnetic Resonance Fingerprinting for Quantitative Biomedical Imaging Applications: A Review.Bioengineering (Basel). 2024 Feb 28;11(3):236. doi: 10.3390/bioengineering11030236. Bioengineering (Basel). 2024. PMID: 38534511 Free PMC article. Review.
-
Fingerprinting MINOCA: Unraveling Clues With Quantitative CMR.JACC Case Rep. 2023 Feb 1;7:101722. doi: 10.1016/j.jaccas.2022.101722. eCollection 2023 Feb 1. JACC Case Rep. 2023. PMID: 36776793 Free PMC article.
-
Simultaneous multi-parametric acquisition and reconstruction techniques in cardiac magnetic resonance imaging: Basic concepts and status of clinical development.Front Cardiovasc Med. 2022 Oct 6;9:953823. doi: 10.3389/fcvm.2022.953823. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36277755 Free PMC article. Review.
References
-
- Messroghli DR et al., “Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI),” J. Cardiovasc. Magn. Reson, vol. 19, no. 1, p. 75, October. 2017, doi: 10.1186/s12968-017-0389-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

