The role of biomarkers and neuroimaging in ischemic/hemorrhagic risk assessment for cardiovascular/cerebrovascular disease prevention
- PMID: 33632452
- PMCID: PMC9044196
- DOI: 10.1016/B978-0-12-819814-8.00021-4
The role of biomarkers and neuroimaging in ischemic/hemorrhagic risk assessment for cardiovascular/cerebrovascular disease prevention
Abstract
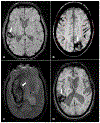
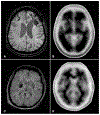
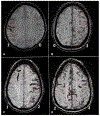
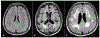
Stroke prevention in patients with atrial fibrillation is arguably one of the fastest developing areas in preventive medicine. The increasing use of direct oral anticoagulants and nonpharmacologic methods such as left atrial appendage closure for stroke prevention in these patients has increased clinicians' options for optimal care. Platelet antiaggregants are also commonly used in other ischemic cardiovascular and or cerebrovascular conditions. Long term use of oral anticoagulants for atrial fibrillation is associated with elevated risks of major bleeds including especially brain hemorrhages, which are known to have extremely poor outcomes. Neuroimaging and other biomarkers have been validated to stratify brain hemorrhage risk among older adults. A thorough understanding of these biomarkers is essential for selection of appropriate anticoagulant or left atrial appendage closure for stroke prevention in patients with atrial fibrillation. This article will address advances in the stratification of ischemic and hemorrhagic stroke risk among patients with atrial fibrillation and other conditions.
Keywords: Anticoagulation; Antithrombotic; Cerebral amyloid angiopathy; Hypertensive cerebral hemorrhage; Intracerebral hemorrhage; Platelet antiaggregants; Risk scores; Stroke prevention.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures
References
-
- Bekwelem W, Connolly SJ, Halperin JL et al. (2015). Extracranial systemic embolic events in patients with nonvalvular atrial fibrillation: incidence, risk factors, and outcomes. Circulation 132: 796–803. - PubMed
-
- Blackshear JL, Odell JA (1996). Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 61: 755–759. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

