In Vivo Functional Effects of CYP2C9 M1L, a Novel and Common Variant in the Yup'ik Alaska Native Population
- PMID: 33632714
- PMCID: PMC8008381
- DOI: 10.1124/dmd.120.000301
In Vivo Functional Effects of CYP2C9 M1L, a Novel and Common Variant in the Yup'ik Alaska Native Population
Abstract
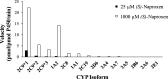
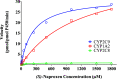
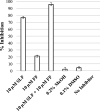
Alaska Native people are under-represented in genetic research but have unique gene variation that may critically impact their response to pharmacotherapy. Full resequencing of CYP2C9 in a cross-section of this population identified CYP2C9 Met1Leu (M1L), a novel, relatively common single nucleotide polymorphism hypothesized to confer CYP2C9 poor metabolizer phenotype by disrupting the start codon. M1L is present at a minor allele frequency of 6.3% in Yup'ik Alaska Native people and thus can contribute to the risk of an adverse drug response from narrow-therapeutic-index CYP2C9 substrates such as (S)-warfarin. This study's objective was to characterize the catalytic efficiency of the Leu1 variant enzyme in vivo by evaluating the pharmacokinetic behavior of naproxen, a probe substrate for CYP2C9 activity, in genotyped Yup'ik participants. We first confirmed the selectivity of (S)-naproxen O-demethylation by CYP2C9 using activity-phenotyped human liver microsomes and selective cytochrome P450 inhibitors and then developed and validated a novel liquid chromatography mass spectrometry method for simultaneous quantification of (S)-naproxen, (S)-O-desmethylnaproxen, and naproxen acyl glucuronide in human urine. The average ratio of (S)-O-desmethylnaproxen to unchanged (S)-naproxen in urine was 18.0 ± 8.0 (n = 11) for the homozygous CYP2C9Met1 reference group and 10.3 ± 6.6 (n = 11) for the Leu1 variant carrier group (P = 0.011). The effect of M1L variation on CYP2C9 function and its potential to alter the pharmacokinetics of drugs metabolized by the enzyme has clinical implications and should be included in a variant screening panel when pharmacogenetic testing in the Alaska Native population is warranted. SIGNIFICANCE STATEMENT: The novel CYP2C9 Met1Leu variant in Alaska Native people was recently identified. This study validated (S)-naproxen as a CYP2C9 probe substrate to characterize the in vivo functional activity of the CYP2C9 Leu1 variant. The results of this pharmacogenetic-pharmacokinetic study suggest that the CYP2C9 Leu1 variant exhibits loss of enzyme activity. This finding may be important to consider when administering narrow-therapeutic-index medications metabolized by CYP2C9 and also compels further investigation to characterize novel genetic variation in understudied populations.
Copyright © 2021 by The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Variation in genes controlling warfarin disposition and response in American Indian and Alaska Native people: CYP2C9, VKORC1, CYP4F2, CYP4F11, GGCX.Pharmacogenet Genomics. 2015 Jul;25(7):343-353. doi: 10.1097/FPC.0000000000000143. Pharmacogenet Genomics. 2015. PMID: 25946405 Free PMC article.
-
Heterologous Expression and Functional Characterization of Novel CYP2C9 Variants Identified in the Alaska Native People.J Pharmacol Exp Ther. 2020 Aug;374(2):233-240. doi: 10.1124/jpet.120.265850. Epub 2020 May 18. J Pharmacol Exp Ther. 2020. PMID: 32423989 Free PMC article.
-
Characterization of CYP3A pharmacogenetic variation in American Indian and Alaska Native communities, targeting CYP3A4*1G allele function.Clin Transl Sci. 2021 Jul;14(4):1292-1302. doi: 10.1111/cts.12970. Epub 2021 Jan 27. Clin Transl Sci. 2021. PMID: 33503331 Free PMC article.
-
Pharmacogenetics of warfarin elimination and its clinical implications.Clin Pharmacokinet. 2001;40(8):587-603. doi: 10.2165/00003088-200140080-00003. Clin Pharmacokinet. 2001. PMID: 11523725 Review.
-
Molecular functionality of CYP2C9 polymorphisms and their influence on drug therapy.Drug Metabol Drug Interact. 2014;29(4):211-20. doi: 10.1515/dmdi-2014-0001. Drug Metabol Drug Interact. 2014. PMID: 24825094 Review.
Cited by
-
Implementing community-engaged pharmacogenomics in Indigenous communities.Nat Commun. 2024 Jan 31;15(1):920. doi: 10.1038/s41467-024-45032-5. Nat Commun. 2024. PMID: 38296967 Free PMC article.
-
CYP2C9 Polymorphism Influence in PK/PD Model of Naproxen and 6-O-Desmethylnaproxen in Oral Fluid.Metabolites. 2022 Nov 13;12(11):1106. doi: 10.3390/metabo12111106. Metabolites. 2022. PMID: 36422246 Free PMC article.
References
-
- Bae JW, Kim JH, Choi CI, Kim MJ, Kim HJ, Byun SA, Chang YS, Jang CG, Park YS, Lee SY (2009) Effect of CYP2C9*3 allele on the pharmacokinetics of naproxen in Korean subjects. Arch Pharm Res 32:269–273. - PubMed
-
- Becker ML, Visser LE, Trienekens PH, Hofman A, van Schaik RH, Stricker BH (2008) Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther 83:288–292. - PubMed
-
- Caudle KE, Rettie AE, Whirl-Carrillo M, Smith LH, Mintzer S, Lee MT, Klein TE, Callaghan JT, Clinical Pharmacogenetics Implementation Consortium (2014) Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther 96:542–548. - PMC - PubMed
-
- Center for Drug Evaluation and Research (2005) Naproxen sodium (220 mg), Clinical Pharmacology and Biopharmaceutics Review(s), New Drug Application 21-920.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

