Repeatability and sensitivity to change of non-invasive end points in PAH: the RESPIRE study
- PMID: 33632769
- PMCID: PMC8461450
- DOI: 10.1136/thoraxjnl-2020-216078
Repeatability and sensitivity to change of non-invasive end points in PAH: the RESPIRE study
Abstract
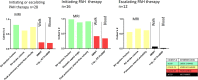
End points that are repeatable and sensitive to change are important in pulmonary arterial hypertension (PAH) for clinical practice and trials of new therapies. In 42 patients with PAH, test-retest repeatability was assessed using the intraclass correlation coefficient and treatment effect size using Cohen's d statistic. Intraclass correlation coefficients demonstrated excellent repeatability for MRI, 6 min walk test and log to base 10 N-terminal pro-brain natriuretic peptide (log10NT-proBNP). The treatment effect size for MRI-derived right ventricular ejection fraction was large (Cohen's d 0.81), whereas the effect size for the 6 min walk test (Cohen's d 0.22) and log10NT-proBNP (Cohen's d 0.20) were fair. This study supports further evaluation of MRI as a non-invasive end point for clinical assessment and PAH therapy trials.Trial registration number NCT03841344.
Keywords: imaging/CT MRI etc; primary pulmonary hypertension.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: FW, LK and AC are employees and shareholders of GlaxoSmithKline. AS is the principal investigator for the collaborative research grant from GlaxoSmithKline that funded this study. AS has undertaken consultancy work for General Electric and Actelion Pharmaceuticals. RC has received fees for lecturing and participation in advisory boards, from Actelion, Bayer, GSK and MSD. DGK has received fees for lecturing and participation in advisory boards, from Actelion, Bayer, GSK and MSD and fees for participation in Steering Committees for Actelion.
Figures

References
-
- Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint Task force for the diagnosis and treatment of pulmonary hypertension of the European Society of cardiology (ESC) and the European respiratory Society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), International Society for heart and lung transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
