Use of a Self-Delivering Anti-CCL3 FANA Oligonucleotide as an Innovative Approach to Target Inflammation after Spinal Cord Injury
- PMID: 33632814
- PMCID: PMC7986543
- DOI: 10.1523/ENEURO.0338-20.2021
Use of a Self-Delivering Anti-CCL3 FANA Oligonucleotide as an Innovative Approach to Target Inflammation after Spinal Cord Injury
Abstract
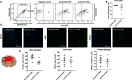
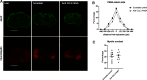
Secondary damage after spinal cord injury (SCI) occurs because of a sequence of events after the initial injury, including exacerbated inflammation that contributes to increased lesion size and poor locomotor recovery. Thus, mitigating secondary damage is critical to preserve neural tissue and improve neurologic outcome. In this work, we examined the therapeutic potential of a novel antisense oligonucleotide (ASO) with special chemical modifications [2'-deoxy-2-fluoro-D-arabinonucleic acid (FANA) ASO] for specifically inhibiting an inflammatory molecule in the injured spinal cord. The chemokine CCL3 plays a complex role in the activation and attraction of immune cells and is upregulated in the injured tissue after SCI. We used specific FANA ASO to inhibit CCL3 in a contusive mouse model of murine SCI. Our results show that self-delivering FANA ASO molecules targeting the chemokine CCL3 penetrate the spinal cord lesion site and suppress the expression of CCL3 transcripts. Furthermore, they reduce other proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukin (IL)-1β after SCI. In summary, we demonstrate for the first time the potential of FANA ASO molecules to penetrate the spinal cord lesion site to specifically inhibit CCL3, reducing proinflammatory cytokines and improve functional recovery after SCI. This novel approach may be used in new treatment strategies for SCI and other pathologic conditions of the CNS.
Keywords: CCL3; FANA ASO; inflammation; novel RNA inhibitor; secondary damage; spinal cord injury.
Copyright © 2021 Pelisch et al.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
