Time Course of Alterations in Adult Spinal Motoneuron Properties in the SOD1(G93A) Mouse Model of ALS
- PMID: 33632815
- PMCID: PMC8009670
- DOI: 10.1523/ENEURO.0378-20.2021
Time Course of Alterations in Adult Spinal Motoneuron Properties in the SOD1(G93A) Mouse Model of ALS
Erratum in
-
Erratum: Huh et al., "Time Course of Alterations in Adult Spinal Motoneuron Properties in the SOD1(G93A) Mouse Model of ALS".eNeuro. 2023 Oct 13;10(10):ENEURO.0370-23.2023. doi: 10.1523/ENEURO.0370-23.2023. Print 2023 Oct. eNeuro. 2023. PMID: 37833071 Free PMC article. No abstract available.
Abstract
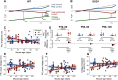
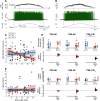
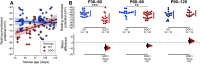
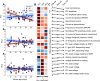
Although amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease, motoneuron electrical properties are already altered during embryonic development. Motoneurons must therefore exhibit a remarkable capacity for homeostatic regulation to maintain a normal motor output for most of the life of the patient. In the present article, we demonstrate how maintaining homeostasis could come at a very high cost. We studied the excitability of spinal motoneurons from young adult SOD1(G93A) mice to end-stage. Initially, homeostasis is highly successful in maintaining their overall excitability. This initial success, however, is achieved by pushing some cells far above the normal range of passive and active conductances. As the disease progresses, both passive and active conductances shrink below normal values in the surviving cells. This shrinkage may thus promote survival, implying the previously large values contribute to degeneration. These results support the hypothesis that motoneuronal homeostasis may be "hypervigilant" in ALS and a source of accumulating stress.
Keywords: ALS; electrophysiology; homeostasis; in vivo recording; motor neuron; spinal cord.
Copyright © 2021 Huh et al.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
