Phenobarbital and Clonidine as Secondary Medications for Neonatal Opioid Withdrawal Syndrome
- PMID: 33632932
- PMCID: PMC7919109
- DOI: 10.1542/peds.2020-017830
Phenobarbital and Clonidine as Secondary Medications for Neonatal Opioid Withdrawal Syndrome
Abstract
Background and objectives: Despite the neonatal opioid withdrawal syndrome (NOWS) epidemic in the United States, evidence is limited for pharmacologic management when first-line opioid medications fail to control symptoms. The objective with this study was to evaluate outcomes of infants receiving secondary therapy with phenobarbital compared with clonidine, in combination with morphine, for the treatment of NOWS.
Methods: We performed a retrospective cohort study of infants with NOWS from 30 hospitals. The primary outcome measures were the length of hospital stay, duration of opioid treatment, and peak morphine dose. Outcomes were compared by group by using analysis of variance and multivariable linear regression controlling for relevant confounders.
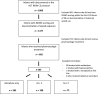
Results: Of 563 infants with NOWS treated with morphine, 32% (n = 180) also received a secondary medication. Seventy-two received phenobarbital and 108 received clonidine. After adjustment for covariates, length of hospital stay was 10 days shorter, and, in some models, duration of morphine treatment was 7.5 days shorter in infants receiving phenobarbital compared with those receiving clonidine, with no difference in peak morphine dose. Infants were more likely to be discharged from the hospital on phenobarbital than clonidine (78% vs 29%, P < .0001).
Conclusions: Among infants with NOWS receiving morphine and secondary therapy, those treated with phenobarbital had shorter length of hospital stay and shorter morphine treatment duration than clonidine-treated infants but were discharged from the hospital more often on secondary medication. Further investigation is warranted to determine if the benefits of shorter hospital stay and shorter duration of morphine therapy justify the possible neurodevelopmental consequences of phenobarbital use in infants with NOWS.
Copyright © 2021 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Whalen is a codeveloper of the ESC Care Tool; Children’s Hospital at Dartmouth-Hitchcock is one of the institutions on the ESC Care Tool copyright; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures
References
-
- Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics . Neonatal drug withdrawal. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e540 - PubMed
-
- Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17 - PubMed
-
- Hall ES, Wexelblatt SL, Crowley M, et al. .; OCHNAS Consortium . A multicenter cohort study of treatments and hospital outcomes in neonatal abstinence syndrome. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e527 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UG1 OD024945/OD/NIH HHS/United States
- U2C OD023375/OD/NIH HHS/United States
- UG1 OD024943/OD/NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UG1 OD024947/OD/NIH HHS/United States
- UG1 OD024958/OD/NIH HHS/United States
- UG1 HD090907/HD/NICHD NIH HHS/United States
- UG1 OD024946/OD/NIH HHS/United States
- U10 HD036790/HD/NICHD NIH HHS/United States
- UG1 HD068278/HD/NICHD NIH HHS/United States
- UG1 OD024953/OD/NIH HHS/United States
- UG1 OD024950/OD/NIH HHS/United States
- UG1 OD024952/OD/NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- UG1 OD024951/OD/NIH HHS/United States
- UG1 OD024948/OD/NIH HHS/United States
- UG1 OD024959/OD/NIH HHS/United States
- UG1 HD090875/HD/NICHD NIH HHS/United States
- UG1 OD024942/OD/NIH HHS/United States
- U24 OD024957/OD/NIH HHS/United States
- UG1 OD024954/OD/NIH HHS/United States
- UG1 OD024944/OD/NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- UG1 OD024956/OD/NIH HHS/United States
- UG1 OD024949/OD/NIH HHS/United States
- UG1 OD024955/OD/NIH HHS/United States
- U10 HD053089/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources