Brief Report: Switching to DOR/3TC/TDF Maintains HIV-1 Virologic Suppression Through Week 144 in the DRIVE-SHIFT Trial
- PMID: 33633036
- PMCID: PMC8126485
- DOI: 10.1097/QAI.0000000000002642
Brief Report: Switching to DOR/3TC/TDF Maintains HIV-1 Virologic Suppression Through Week 144 in the DRIVE-SHIFT Trial
Abstract
Background: In the primary analysis of the DRIVE-SHIFT trial, switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintained suppression of HIV-1 through week 48. Here, we present long-term efficacy and safety outcomes through week 144 of the DRIVE-SHIFT trial.
Methods: This phase 3, randomized, open-label trial evaluated switching from a stable antiretroviral regimen to once-daily DOR/3TC/TDF in adults with HIV-1 suppressed for ≥6 months and no previous virologic failure. Participants switched at day 1 [immediate switch group (ISG); n = 447] or week 24 [delayed switch group (DSG); n = 209]. Nine ISG participants who completed week 48 but did not enter extension-1 were excluded from week 144 efficacy analyses.
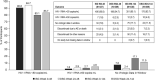
Results: At week 144, HIV-1 RNA <50 copies/mL was maintained in 80.1% of the ISG (351/438) and 83.7% of the DSG (175/209), while 2.7% (12/438) and 4.8% (10/209), respectively, had HIV-1 RNA ≥50 copies/mL (Food and Drug Administration Snapshot approach). Protocol-defined virologic failure after switch occurred in 2.1% of ISG (9/438) and 3.3% of DSG (7/209); no viral resistance to doravirine was detected in 4 participants with samples available. Reductions in fasting lipids were observed at 24 weeks after switch and maintained through week 144. The mean weight change from switch to week 144 was +1.4 kg for ISG and +1.2 kg for DSG. The most common adverse events were nasopharyngitis (16.2%), headache (12.3%), and diarrhea (9.1%). Overall, 4.1% discontinued because of adverse events, and no deaths occurred.
Conclusions: These results confirm that switching to once-daily DOR/3TC/TDF is a generally well-tolerated option for maintaining viral suppression in adults considering a change in therapy.
Registration: ClinicalTrials.gov NCT02397096.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
P.K. is on advisory boards for ViiV, Janssen, Merck, and Theratechnologies; has received grants from Merck, ViiV, Gilead, and Theratechnologies; and owns stock in Johnson & Johnson, Gilead, Merck, Pfizer, and GSK. J.-M.M. has participated in advisory boards for Gilead, MSD, ViiV, and Sanofi. G.R. has received consulting/advisor fees from AbbVie, Gilead, MSD, and ViiV, and has board membership with AbbVie, Gilead, MSD, and ViiV. P.C. is an advisory board member for MSD and ViiV and has received research grants from AbbVie, MSD, and ViiV. H.W., Z.J.X., C.M., P.S., and W.G. are current employees of MSD. The remaining authors have no conflicts of interest to declare.
Figures

Comment in
-
Real-Life Safety of Doravirine in Treatment-Experienced, Virologically Suppressed PLWHIV.J Acquir Immune Defic Syndr. 2021 Sep 1;88(1):e5-e6. doi: 10.1097/QAI.0000000000002730. J Acquir Immune Defic Syndr. 2021. PMID: 34397747 No abstract available.
References
-
- Behm MO, Yee KL, Liu R, et al. The effect of food on doravirine bioavailability: results from two pharmacokinetic studies in healthy subjects. Clin Drug Investig. 2017;37:571–579. - PubMed
-
- Anderson MS, Kaufman D, Castronuovo P, et al. Effect of doravirine (MK-1439) on the pharmacokinetics of an oral contraceptive (ethinyl estradiol and levonorgestrel). Rev Antivir Ther Infect Dis. 2015;4:63–64.
-
- Khalilieh SG, Yee KL, Sanchez RI, et al. A study to evaluate doravirine pharmacokinetics when co-administered with acid-reducing agents. J Clin Pharmacol. 2019;59:1093–1098. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

