Repair of full-thickness articular cartilage defects using IEIK13 self-assembling peptide hydrogel in a non-human primate model
- PMID: 33633122
- PMCID: PMC7907267
- DOI: 10.1038/s41598-021-83208-x
Repair of full-thickness articular cartilage defects using IEIK13 self-assembling peptide hydrogel in a non-human primate model
Abstract
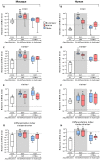
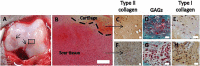
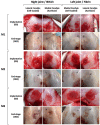
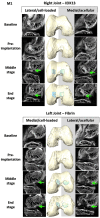
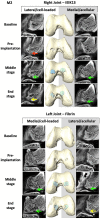
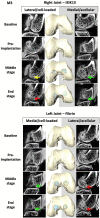
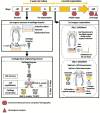
Articular cartilage is built by chondrocytes which become less active with age. This declining function of the chondrocytes, together with the avascular nature of the cartilage, impedes the spontaneous healing of chondral injuries. These lesions can progress to more serious degenerative articular conditions as in the case of osteoarthritis. As no efficient cure for cartilage lesions exist yet, cartilage tissue engineering has emerged as a promising method aiming at repairing joint defects and restoring articular function. In the present work, we investigated if a new self-assembling peptide (referred as IEIK13), combined with articular chondrocytes treated with a chondrogenic cocktail (BMP-2, insulin and T3, designated BIT) could be efficient to restore full-thickness cartilage defects induced in the femoral condyles of a non-human primate model, the cynomolgus monkey. First, in vitro molecular studies indicated that IEIK13 was efficient to support production of cartilage by monkey articular chondrocytes treated with BIT. In vivo, cartilage implant integration was monitored non-invasively by contrast-enhanced micro-computed tomography, and then by post-mortem histological analysis and immunohistochemical staining of the condyles collected 3 months post-implantation. Our results revealed that the full-thickness cartilage injuries treated with either IEIK13 implants loaded with or devoid of chondrocytes showed similar cartilage-characteristic regeneration. This pilot study demonstrates that IEIK13 can be used as a valuable scaffold to support the in vitro activity of articular chondrocytes and the repair of articular cartilage defects, when implanted alone or with chondrocytes.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration.Theranostics. 2018 Oct 5;8(18):5039-5058. doi: 10.7150/thno.26981. eCollection 2018. Theranostics. 2018. PMID: 30429885 Free PMC article.
-
Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs.Int J Mol Sci. 2019 Feb 2;20(3):653. doi: 10.3390/ijms20030653. Int J Mol Sci. 2019. PMID: 30717402 Free PMC article.
-
Combination of bioactive factors and IEIK13 self-assembling peptide hydrogel promotes cartilage matrix production by human nasal chondrocytes.J Biomed Mater Res A. 2019 Apr;107(4):893-903. doi: 10.1002/jbm.a.36612. Epub 2019 Jan 31. J Biomed Mater Res A. 2019. PMID: 30650239
-
Cartilage Tissue Regeneration: The Roles of Cells, Stimulating Factors and Scaffolds.Curr Stem Cell Res Ther. 2018;13(7):547-567. doi: 10.2174/1574888X12666170608080722. Curr Stem Cell Res Ther. 2018. PMID: 28595567 Review.
-
Animal Models Used for Testing Hydrogels in Cartilage Regeneration.Curr Stem Cell Res Ther. 2018;13(7):517-525. doi: 10.2174/1574888X13666180514123103. Curr Stem Cell Res Ther. 2018. PMID: 29756585 Review.
Cited by
-
Recent Biomimetic Approaches for Articular Cartilage Tissue Engineering and Their Clinical Applications: Narrative Review of the Literature.Adv Orthop. 2022 Apr 22;2022:8670174. doi: 10.1155/2022/8670174. eCollection 2022. Adv Orthop. 2022. PMID: 35497390 Free PMC article. Review.
-
Peptide-Based Hydrogels: Template Materials for Tissue Engineering.J Funct Biomater. 2023 Apr 19;14(4):233. doi: 10.3390/jfb14040233. J Funct Biomater. 2023. PMID: 37103323 Free PMC article. Review.
-
Advances in Peptide-Based Hydrogel for Tissue Engineering.Polymers (Basel). 2023 Feb 21;15(5):1068. doi: 10.3390/polym15051068. Polymers (Basel). 2023. PMID: 36904309 Free PMC article. Review.
-
Peptides for Targeting Chondrogenic Induction and Cartilage Regeneration in Osteoarthritis.Cartilage. 2024 Sep 18:19476035241276406. doi: 10.1177/19476035241276406. Online ahead of print. Cartilage. 2024. PMID: 39291443 Free PMC article.
-
Functional Nanomaterials for the Treatment of Osteoarthritis.Int J Nanomedicine. 2024 Jul 4;19:6731-6756. doi: 10.2147/IJN.S465243. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38979531 Free PMC article. Review.
References
-
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou R, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/joca.2014.01.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

