Interaction of Plasmodium falciparum apicortin with α- and β-tubulin is critical for parasite growth and survival
- PMID: 33633135
- PMCID: PMC7907060
- DOI: 10.1038/s41598-021-83513-5
Interaction of Plasmodium falciparum apicortin with α- and β-tubulin is critical for parasite growth and survival
Abstract
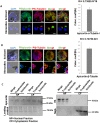
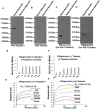
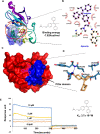
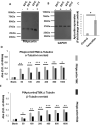
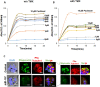
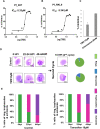
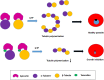
Cytoskeletal structures of Apicomplexan parasites are important for parasite replication, motility, invasion to the host cell and survival. Apicortin, an Apicomplexan specific protein appears to be a crucial factor in maintaining stability of the parasite cytoskeletal assemblies. However, the function of apicortin, in terms of interaction with microtubules still remains elusive. Herein, we have attempted to elucidate the function of Plasmodium falciparum apicortin by monitoring its interaction with two main components of parasite microtubular structure, α-tubulin-I and β-tubulin through in silico and in vitro studies. Further, a p25 domain binding generic drug Tamoxifen (TMX), was used to disrupt PfApicortin-tubulin interactions which led to the inhibition in growth and progression of blood stage life cycle of P. falciparum.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen-Host Interaction.Trop Med Infect Dis. 2021 Jun 30;6(3):118. doi: 10.3390/tropicalmed6030118. Trop Med Infect Dis. 2021. PMID: 34209186 Free PMC article. Review.
-
Targeted repression of Plasmodium apicortin by host microRNA impairs malaria parasite growth and invasion.Dis Model Mech. 2020 Jun 3;13(6):dmm042820. doi: 10.1242/dmm.042820. Dis Model Mech. 2020. PMID: 32493727 Free PMC article.
-
Purification of functional Plasmodium falciparum tubulin allows for the identification of parasite-specific microtubule inhibitors.Curr Biol. 2022 Feb 28;32(4):919-926.e6. doi: 10.1016/j.cub.2021.12.049. Epub 2022 Jan 19. Curr Biol. 2022. PMID: 35051355
-
Isotype expression, post-translational modification and stage-dependent production of tubulins in erythrocytic Plasmodium falciparum.Int J Parasitol. 2008 Apr;38(5):527-39. doi: 10.1016/j.ijpara.2007.09.005. Epub 2007 Sep 29. Int J Parasitol. 2008. PMID: 17977543
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
Cited by
-
Host-Erythrocytic Sphingosine-1-Phosphate Regulates Plasmodium Histone Deacetylase Activity and Exhibits Epigenetic Control over Cell Death and Differentiation.Microbiol Spectr. 2023 Feb 6;11(2):e0276622. doi: 10.1128/spectrum.02766-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36744922 Free PMC article.
-
Genomic epidemiology of Plasmodium knowlesi reveals putative genetic drivers of adaptation in Malaysia.PLoS Negl Trop Dis. 2025 Mar 12;19(3):e0012885. doi: 10.1371/journal.pntd.0012885. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40072967 Free PMC article.
-
Apicortin, a Constituent of Apicomplexan Conoid/Apical Complex and Its Tentative Role in Pathogen-Host Interaction.Trop Med Infect Dis. 2021 Jun 30;6(3):118. doi: 10.3390/tropicalmed6030118. Trop Med Infect Dis. 2021. PMID: 34209186 Free PMC article. Review.
-
Apicortin, a Putative Apicomplexan-Specific Protein, Is Present in Deep-Branching Opisthokonts.Biology (Basel). 2025 May 28;14(6):620. doi: 10.3390/biology14060620. Biology (Basel). 2025. PMID: 40563871 Free PMC article.
-
CRISPR/Cas9-engineering of Kell null erythrocytes to unveil host targeted irresistible antimalarial.Commun Biol. 2025 May 11;8(1):730. doi: 10.1038/s42003-025-07968-2. Commun Biol. 2025. PMID: 40350476 Free PMC article.
References
-
- Bloland PB, Organization WH. Drug Resistance in Malaria. Geneva: World Health Organization; 2001.
-
- Kappes, B. & Rohrbach, P. Microtubule inhibitors as a potential treatment for malaria. Future Microbiology2(4), 409–423 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

