SPARC coordinates extracellular matrix remodeling and efficient recruitment to and migration of antigen-specific T cells in the brain following infection
- PMID: 33633185
- PMCID: PMC7907143
- DOI: 10.1038/s41598-021-83952-0
SPARC coordinates extracellular matrix remodeling and efficient recruitment to and migration of antigen-specific T cells in the brain following infection
Abstract
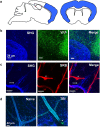
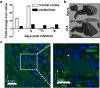
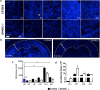
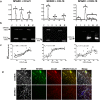
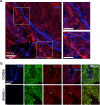
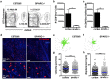
Central nervous system (CNS) injury and infection can result in profound tissue remodeling in the brain, the mechanism and purpose of which is poorly understood. Infection with the protozoan parasite Toxoplasma gondii causes chronic infection and inflammation in the brain parenchyma. Control of parasite replication requires the continuous presence of IFNγ-producing T cells to keep T. gondii in its slowly replicating cyst form. During infection, a network of extracellular matrix fibers, revealed using multiphoton microscopy, forms in the brain. The origin and composition of these structures are unknown but the fibers have been observed to act as a substrate for migrating T cells. In this study, we show a critical regulator of extracellular matrix (ECM) remodeling, Secreted Protein, Acidic, Rich in Cysteine (SPARC), is upregulated in the brain during the early phases of infection in the frontal cortex. In the absence of SPARC, a reduced and disordered fibrous network, increased parasite burden, and reduced antigen-specific T cell entry into the brain points to a role for SPARC in T cell recruitment to and migration within the brain. We also report SPARC can directly bind to CCR7 ligands CCL19 and CCL21 but not CXCL10, and enhance migration toward a chemokine gradient. Measurement of T cell behavior points to tissue remodeling being important for access of immune cells to the brain and facilitating cellular locomotion. Together, these data identify SPARC as an important regulatory component of immune cell trafficking and access to the inflamed CNS.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
CCR7-dependent immunity during acute Toxoplasma gondii infection.Infect Immun. 2010 May;78(5):2257-63. doi: 10.1128/IAI.01314-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194594 Free PMC article.
-
CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection.Brain Behav Immun. 2011 Jul;25(5):883-96. doi: 10.1016/j.bbi.2010.09.014. Epub 2010 Sep 22. Brain Behav Immun. 2011. PMID: 20868739 Free PMC article.
-
Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone.PLoS One. 2011;6(9):e23880. doi: 10.1371/journal.pone.0023880. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949685 Free PMC article.
-
The Influence of the Extracellular Matrix in Inflammation: Findings from the SPARC-Null Mouse.Anat Rec (Hoboken). 2020 Jun;303(6):1624-1629. doi: 10.1002/ar.24133. Epub 2019 Apr 24. Anat Rec (Hoboken). 2020. PMID: 30980479 Free PMC article. Review.
-
Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC).J Mol Cell Cardiol. 2010 Mar;48(3):544-9. doi: 10.1016/j.yjmcc.2009.06.018. Epub 2009 Jul 3. J Mol Cell Cardiol. 2010. PMID: 19577572 Free PMC article. Review.
Cited by
-
Cell-Instructive Biomaterials with Native-Like Biochemical Complexity.Annu Rev Biomed Eng. 2025 May;27(1):185-209. doi: 10.1146/annurev-bioeng-120823-020209. Epub 2025 Jan 28. Annu Rev Biomed Eng. 2025. PMID: 39874600 Review.
-
Single-Cell Profiling Indicates a Proinflammatory Role of Meningeal Ectopic Lymphoid Tissue in Experimental Autoimmune Encephalomyelitis.Neurol Neuroimmunol Neuroinflamm. 2024 Jan;11(1):e200185. doi: 10.1212/NXI.0000000000200185. Epub 2023 Dec 15. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38100739 Free PMC article.
-
Diverse Subpopulations of Reactive Astrocytes Following Chronic Toxoplasma Infection.Glia. 2025 Oct;73(10):2003-2024. doi: 10.1002/glia.70053. Epub 2025 Jul 9. Glia. 2025. PMID: 40635167 Free PMC article.
-
From the immune system to mood disorders especially induced by Toxoplasma gondii: CD4+ T cell as a bridge.Front Cell Infect Microbiol. 2023 Apr 3;13:1078984. doi: 10.3389/fcimb.2023.1078984. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37077528 Free PMC article. Review.
-
Effects of exercise and doxorubicin on acute diaphragm neuromuscular transmission failure.Exp Neurol. 2024 Aug;378:114818. doi: 10.1016/j.expneurol.2024.114818. Epub 2024 May 21. Exp Neurol. 2024. PMID: 38782352 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

