Characterization of mitochondrial health from human peripheral blood mononuclear cells to cerebral organoids derived from induced pluripotent stem cells
- PMID: 33633238
- PMCID: PMC7907388
- DOI: 10.1038/s41598-021-84071-6
Characterization of mitochondrial health from human peripheral blood mononuclear cells to cerebral organoids derived from induced pluripotent stem cells
Abstract
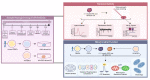
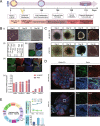
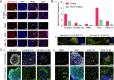
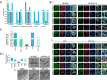
Mitochondrial health plays a crucial role in human brain development and diseases. However, the evaluation of mitochondrial health in the brain is not incorporated into clinical practice due to ethical and logistical concerns. As a result, the development of targeted mitochondrial therapeutics remains a significant challenge due to the lack of appropriate patient-derived brain tissues. To address these unmet needs, we developed cerebral organoids (COs) from induced pluripotent stem cells (iPSCs) derived from human peripheral blood mononuclear cells (PBMCs) and monitored mitochondrial health from the primary, reprogrammed and differentiated stages. Our results show preserved mitochondrial genetics, function and treatment responses across PBMCs to iPSCs to COs, and measurable neuronal activity in the COs. We expect our approach will serve as a model for more widespread evaluation of mitochondrial health relevant to a wide range of human diseases using readily accessible patient peripheral (PBMCs) and stem-cell derived brain tissue samples.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Inducing mononuclear cells of patients with CADASIL to construct a CSVD disease model.Eur J Med Res. 2025 Apr 2;30(1):227. doi: 10.1186/s40001-025-02491-w. Eur J Med Res. 2025. PMID: 40176124 Free PMC article.
-
Characterization of the human induced pluripotent stem cell (iPSC) SZGJMSi004-A line from a 28-year-old Han male patient with depression.Stem Cell Res. 2024 Jun;77:103428. doi: 10.1016/j.scr.2024.103428. Epub 2024 Apr 27. Stem Cell Res. 2024. PMID: 38696853
-
Dynamic Characterization of Structural, Molecular, and Electrophysiological Phenotypes of Human-Induced Pluripotent Stem Cell-Derived Cerebral Organoids, and Comparison with Fetal and Adult Gene Profiles.Cells. 2020 May 23;9(5):1301. doi: 10.3390/cells9051301. Cells. 2020. PMID: 32456176 Free PMC article.
-
Tackling mitochondrial diversity in brain function: from animal models to human brain organoids.Int J Biochem Cell Biol. 2020 Jun;123:105760. doi: 10.1016/j.biocel.2020.105760. Epub 2020 Apr 25. Int J Biochem Cell Biol. 2020. PMID: 32339638 Review.
-
Mitochondrial resetting and metabolic reprogramming in induced pluripotent stem cells and mitochondrial disease modeling.Biochim Biophys Acta. 2016 Apr;1860(4):686-93. doi: 10.1016/j.bbagen.2016.01.009. Epub 2016 Jan 15. Biochim Biophys Acta. 2016. PMID: 26779594 Review.
Cited by
-
Human Brain-Based Models Provide a Powerful Tool for the Advancement of Parkinson's Disease Research and Therapeutic Development.Front Neurosci. 2022 May 16;16:851058. doi: 10.3389/fnins.2022.851058. eCollection 2022. Front Neurosci. 2022. PMID: 35651633 Free PMC article. Review.
-
Metabolic control of induced pluripotency.Front Cell Dev Biol. 2024 Jan 11;11:1328522. doi: 10.3389/fcell.2023.1328522. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38274274 Free PMC article. Review.
-
Inhibition of glycogen synthase kinase 3 by lithium, a mechanism in search of specificity.Front Mol Neurosci. 2022 Nov 24;15:1028963. doi: 10.3389/fnmol.2022.1028963. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36504683 Free PMC article. Review.
-
Optimization of differential filtration-based mitochondrial isolation for mitochondrial transplant to cerebral organoids.Stem Cell Res Ther. 2023 Aug 15;14(1):202. doi: 10.1186/s13287-023-03436-y. Stem Cell Res Ther. 2023. PMID: 37580812 Free PMC article.
-
Linking Mitochondrial Dysfunction, Neurotransmitter, and Neural Network Abnormalities and Mania: Elucidating Neurobiological Mechanisms of the Therapeutic Effect of the Ketogenic Diet in Bipolar Disorder.Biol Psychiatry Cogn Neurosci Neuroimaging. 2025 Mar;10(3):267-277. doi: 10.1016/j.bpsc.2024.07.011. Epub 2024 Jul 23. Biol Psychiatry Cogn Neurosci Neuroimaging. 2025. PMID: 39053576 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

