A glycoside analog of mammalian oligomannose formulated with a TLR4-stimulating adjuvant elicits HIV-1 cross-reactive antibodies
- PMID: 33633304
- PMCID: PMC7907241
- DOI: 10.1038/s41598-021-84116-w
A glycoside analog of mammalian oligomannose formulated with a TLR4-stimulating adjuvant elicits HIV-1 cross-reactive antibodies
Abstract
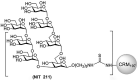
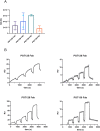
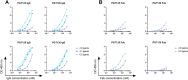
The occurrence of oligomannose-specific broadly neutralizing antibodies (bnAbs) has spurred efforts to develop immunogens that can elicit similar antibodies. Here, we report on the antigenicity and immunogenicity of a CRM197-conjugate of a previously reported oligomannose mimetic. Oligomannose-specific bnAbs that are less dependent on interactions with the HIV envelope protein sequence showed strong binding to the glycoconjugates, with affinities approximating those reported for their cognate epitope. The glycoconjugate is also recognized by inferred germline precursors of oligomannose-specific bnAbs, albeit with the expected low avidity, supporting its potential as an immunogen. Immunization of human-antibody transgenic mice revealed that only a TLR4-stimulating adjuvant formulation resulted in antibodies able to bind a panel of recombinant HIV trimers. These antibodies bound at relatively modest levels, possibly explaining their inability to neutralize HIV infectivity. Nevertheless, these findings contribute further to understanding conditions for eliciting HIV-cross-reactive oligomannose-specific antibodies and inform on next steps for improving on the elicited response.
Conflict of interest statement
The authors declare no competing interests.
Figures
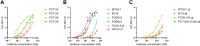

Similar articles
-
Bacterially derived synthetic mimetics of mammalian oligomannose prime antibody responses that neutralize HIV infectivity.Nat Commun. 2017 Nov 17;8(1):1601. doi: 10.1038/s41467-017-01640-y. Nat Commun. 2017. PMID: 29150603 Free PMC article.
-
Toward a carbohydrate-based HIV-1 vaccine: synthesis and immunological studies of oligomannose-containing glycoconjugates.Bioconjug Chem. 2006 Mar-Apr;17(2):493-500. doi: 10.1021/bc0502816. Bioconjug Chem. 2006. PMID: 16536482
-
Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D.J Virol. 2014 Mar;88(5):2645-57. doi: 10.1128/JVI.03228-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352455 Free PMC article.
-
Potent Induction of Envelope-Specific Antibody Responses by Virus-Like Particle Immunogens Based on HIV-1 Envelopes from Patients with Early Broadly Neutralizing Responses.J Virol. 2022 Jan 12;96(1):e0134321. doi: 10.1128/JVI.01343-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668778 Free PMC article.
-
Targeting broadly neutralizing antibody precursors: a naïve approach to vaccine design.Curr Opin HIV AIDS. 2019 Jul;14(4):294-301. doi: 10.1097/COH.0000000000000548. Curr Opin HIV AIDS. 2019. PMID: 30946041 Review.
Cited by
-
Synthetic Neoglycoconjugates of Hepta- and Nonamannoside Ligands for Eliciting Oligomannose-Specific HIV-1-Neutralizing Antibodies.Chembiochem. 2022 Apr 5;23(7):e202200061. doi: 10.1002/cbic.202200061. Epub 2022 Feb 11. Chembiochem. 2022. PMID: 35104013 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

