Golden berry 4β-hydroxywithanolide E prevents tumor necrosis factor α-induced procoagulant activity with enhanced cytotoxicity against human lung cancer cells
- PMID: 33633307
- PMCID: PMC7907079
- DOI: 10.1038/s41598-021-84207-8
Golden berry 4β-hydroxywithanolide E prevents tumor necrosis factor α-induced procoagulant activity with enhanced cytotoxicity against human lung cancer cells
Abstract
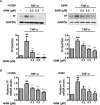
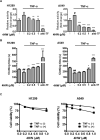
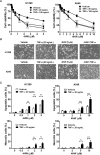
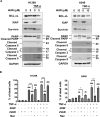
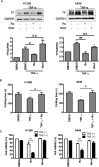
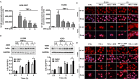
Inflammation in the tumor microenvironment is positively correlated with cancer progression and metastasis as well as the risk of thromboembolism in lung cancer patients. Here we show, in human non-small cell lung cancer (NSCLC) cell lines, the master inflammatory cytokine tumor necrosis factor (TNF-α) induced tissue factor expression and procoagulant activity, and these effects were potently inhibited by 4β-hydroxywithanolide E (4HW), a natural compound isolated from Physalis peruviana. Furthermore, combination of 4HW and TNF-α caused synergistic cytotoxicity against NSCLC cells by inducing caspase-dependent apoptosis. The underlying mechanism by which 4HW reverses the procoagulant effect of TNF-α but enhances its cytotoxic effect appears to be due to inhibition of NF-κB, which is a key switch for both inflammation-induced coagulation and cell survival. Our results suggest that 4HW may have a potential application for treating inflammation-derived cancer progression and cancer-associated hypercoagulable state.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
4beta-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest.BMC Cancer. 2010 Feb 18;10:46. doi: 10.1186/1471-2407-10-46. BMC Cancer. 2010. PMID: 20167063 Free PMC article.
-
The Roles of 4β-Hydroxywithanolide E from Physalis peruviana on the Nrf2-Anti-Oxidant System and the Cell Cycle in Breast Cancer Cells.Am J Chin Med. 2016;44(3):617-36. doi: 10.1142/S0192415X16500348. Epub 2016 Apr 24. Am J Chin Med. 2016. PMID: 27109152
-
Golden berry leaf extract containing withanolides suppresses TNF-α and IL-17 induced IL-6 expression in HeLa Cells.Biosci Biotechnol Biochem. 2023 Aug 23;87(9):972-980. doi: 10.1093/bbb/zbad070. Biosci Biotechnol Biochem. 2023. PMID: 37279446
-
4β-Hydroxywithanolide E from Goldenberry (Whole Fruits of Physalis peruviana L.) as a Promising Agent against Chronic Obstructive Pulmonary Disease.J Nat Prod. 2020 Apr 24;83(4):1217-1228. doi: 10.1021/acs.jnatprod.9b01265. Epub 2020 Mar 11. J Nat Prod. 2020. PMID: 32159343
-
Withanolides derived from Physalis peruviana (Poha) with potential anti-inflammatory activity.Bioorg Med Chem Lett. 2016 Jun 15;26(12):2755-2759. doi: 10.1016/j.bmcl.2016.04.077. Epub 2016 Apr 26. Bioorg Med Chem Lett. 2016. PMID: 27210437
Cited by
-
Impact of daily administration of blackberry extract on gerbil model of transient cerebral ischemia.Acta Cir Bras. 2024 Sep 9;39:e397424. doi: 10.1590/acb397424. eCollection 2024. Acta Cir Bras. 2024. PMID: 39258621 Free PMC article.
-
Hairy root culture: a potent method for improved secondary metabolite production of Solanaceous plants.Front Plant Sci. 2023 Sep 4;14:1197555. doi: 10.3389/fpls.2023.1197555. eCollection 2023. Front Plant Sci. 2023. PMID: 37731987 Free PMC article. Review.
-
Management of triple-negative breast cancer by natural compounds through different mechanistic pathways.Front Genet. 2024 Jul 26;15:1440430. doi: 10.3389/fgene.2024.1440430. eCollection 2024. Front Genet. 2024. PMID: 39130753 Free PMC article. Review.
-
Identification of costimulatory molecule signatures for evaluating prognostic risk in non-small cell lung cancer.Heliyon. 2024 Aug 30;10(17):e36816. doi: 10.1016/j.heliyon.2024.e36816. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39286099 Free PMC article.
-
Recent Studies on Berry Bioactives and Their Health-Promoting Roles.Molecules. 2021 Dec 24;27(1):108. doi: 10.3390/molecules27010108. Molecules. 2021. PMID: 35011338 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

