Development of a Solid Supersaturable Micelle of Revaprazan for Improved Dissolution and Oral Bioavailability Using Box-Behnken Design
- PMID: 33633449
- PMCID: PMC7901570
- DOI: 10.2147/IJN.S298450
Development of a Solid Supersaturable Micelle of Revaprazan for Improved Dissolution and Oral Bioavailability Using Box-Behnken Design
Abstract
Purpose: To enhance the oral bioavailability of revaprazan (RVP), a novel solid, supersaturable micelle (SSuM) was developed.
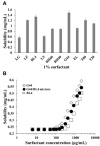
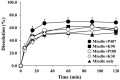
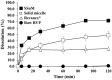
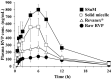
Methods: Surfactants and solid carriers were screened based on a solubility and a flowability test, respectively. Supersaturating agents, including Poloxamer 407 (P407), were screened. The SSuM was optimized using a Box-Behnken design with three independent variables, including Gelucire 44/14:Brij L4 (G44/BL4; X1) and the amounts of Florite PS-10 (FLO; X2) and Vivapur 105 (VP105; X3), and three response variables, ie, dissolution efficiency at 30 min (Y1), dissolution enhancing capacity (Y2), and Carr's index (Y3). The solid state property was evaluated, and a dissolution test was conducted. RVP, Revanex®, solid micelle (P407-free from the composition of SSuM), and SSuM were orally administrated to rats (RVP 20 mg equivalent/kg) for in vivo pharmacokinetic study.
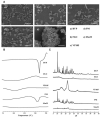
Results: G44 and BL4 showed great solubility, with a critical micelle concentration range of 119.2-333.0 μg/mL. P407 had an excellent supersaturating effect. FLO and VP105 were selected as solid carriers, with a critical solidifying ratio (g/mL) of 0.30 and 0.91, respectively. With optimized values of X1 (-0.41), X2 (0.31), and X3 (-0.78), RVP (200 mg)-containing SSuM consisting of G44 (253.8 mg), BL4 (106.2 mg), FLO (99.3 mg), VP105 (199.8 mg), and P407 (40 mg) was developed, resulting in Y1 (40.3%), Y2 (0.008), and Y3 (12.3%). RVP existed in an amorphous state in the optimized SSuM, and the SSuM formed a nanosized dispersion in the aqueous phase, with approximately 71.7% dissolution at 2 h. The optimized SSuM improved the relative bioavailability of RVP in rats by approximately 478%, 276%, and 161% compared to raw RVP, Revanex®, and solid micelle, respectively.
Conclusion: The optimized SSuM has great potential for the development of solidified formulations of poorly water-soluble drugs with improved oral absorption.
Keywords: Box-Behnken design; dissolution; oral bioavailability; revaprazan; solid micelle; supersaturation.
© 2021 Goo et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Optimization of a solidified micelle formulation for enhanced oral bioavailability of atorvastatin calcium using statistical experimental design.Pharm Dev Technol. 2023 Jun;28(5):479-491. doi: 10.1080/10837450.2023.2208206. Epub 2023 May 5. Pharm Dev Technol. 2023. PMID: 37099663
-
Optimization of solid self-dispersing micelle for enhancing dissolution and oral bioavailability of valsartan using Box-Behnken design.Int J Pharm. 2020 Jul 30;585:119483. doi: 10.1016/j.ijpharm.2020.119483. Epub 2020 May 30. Int J Pharm. 2020. PMID: 32485217
-
Comparison of a revaprazan-loaded solid dispersion, solid SNEDDS and inclusion compound: Physicochemical characterisation and pharmacokinetics.Colloids Surf B Biointerfaces. 2018 Feb 1;162:420-426. doi: 10.1016/j.colsurfb.2017.12.017. Epub 2017 Dec 12. Colloids Surf B Biointerfaces. 2018. PMID: 29248606
-
Spray Congealing: An Emerging Technology to Prepare Solid Dispersions with Enhanced Oral Bioavailability of Poorly Water Soluble Drugs.Molecules. 2019 Sep 25;24(19):3471. doi: 10.3390/molecules24193471. Molecules. 2019. PMID: 31557815 Free PMC article. Review.
-
Self-nanomicellizing solid dispersion: A promising platform for oral drug delivery.Colloids Surf B Biointerfaces. 2024 Sep;241:114057. doi: 10.1016/j.colsurfb.2024.114057. Epub 2024 Jun 24. Colloids Surf B Biointerfaces. 2024. PMID: 38924852 Review.
Cited by
-
The Impact of Process and Formulation Parameters on the Fabrication of Efavirenz Nanosuspension to Improve Drug Solubility and Dissolution.Iran J Pharm Res. 2022 Sep 23;21(1):e129409. doi: 10.5812/ijpr-129409. eCollection 2022 Dec. Iran J Pharm Res. 2022. PMID: 36942076 Free PMC article.
-
Development and evaluation of mPEG-PLLA polymeric micelles encapsulating enrofloxacin for enhanced solubility, bioavailability, and antibacterial performance.Front Vet Sci. 2025 Jul 16;12:1595137. doi: 10.3389/fvets.2025.1595137. eCollection 2025. Front Vet Sci. 2025. PMID: 40740299 Free PMC article.
-
Regeneration of articular cartilage defects: Therapeutic strategies and perspectives.J Tissue Eng. 2023 Mar 31;14:20417314231164765. doi: 10.1177/20417314231164765. eCollection 2023 Jan-Dec. J Tissue Eng. 2023. PMID: 37025158 Free PMC article. Review.
References
-
- Suzuki H. New pharmacological treatments for gastroesophageal reflux: potassium-competitive acid blockers and bile acid sequestrants. NeuroGastroLatam Rev. 2018;2:18–27. doi:10.24875/NGL.18000007 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

