Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS
- PMID: 33633540
- PMCID: PMC7900543
- DOI: 10.3389/fnins.2021.639140
Glymphatic System as a Gateway to Connect Neurodegeneration From Periphery to CNS
Abstract
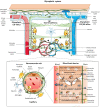
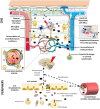
The classic concept of the absence of lymphatic vessels in the central nervous system (CNS), suggesting the immune privilege of the brain in spite of its high metabolic rate, was predominant until recent times. On the other hand, this idea left questioned how cerebral interstitial fluid is cleared of waste products. It was generally thought that clearance depends on cerebrospinal fluid (CSF). Not long ago, an anatomically and functionally discrete paravascular space was revised to provide a pathway for the clearance of molecules drained within the interstitial space. According to this model, CSF enters the brain parenchyma along arterial paravascular spaces. Once mixed with interstitial fluid and solutes in a process mediated by aquaporin-4, CSF exits through the extracellular space along venous paravascular spaces, thus being removed from the brain. This process includes the participation of perivascular glial cells due to a sieving effect of their end-feet. Such draining space resembles the peripheral lymphatic system, therefore, the term "glymphatic" (glial-lymphatic) pathway has been coined. Specific studies focused on the potential role of the glymphatic pathway in healthy and pathological conditions, including neurodegenerative diseases. This mainly concerns Alzheimer's disease (AD), as well as hemorrhagic and ischemic neurovascular disorders; other acute degenerative processes, such as normal pressure hydrocephalus or traumatic brain injury are involved as well. Novel morphological and functional investigations also suggested alternative models to drain molecules through perivascular pathways, which enriched our insight of homeostatic processes within neural microenvironment. Under the light of these considerations, the present article aims to discuss recent findings and concepts on nervous lymphatic drainage and blood-brain barrier (BBB) in an attempt to understand how peripheral pathological conditions may be detrimental to the CNS, paving the way to neurodegeneration.
Keywords: blood–brain barrier; glymphatic system; lymphatic system; neurodegenerative diseases; neurovascular unit.
Copyright © 2021 Natale, Limanaqi, Busceti, Mastroiacovo, Nicoletti, Puglisi-Allegra and Fornai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy.Front Neuroanat. 2017 Nov 7;11:101. doi: 10.3389/fnana.2017.00101. eCollection 2017. Front Neuroanat. 2017. PMID: 29163074 Free PMC article. Review.
-
The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system?Acta Neuropathol. 2018 Mar;135(3):387-407. doi: 10.1007/s00401-018-1812-4. Epub 2018 Feb 10. Acta Neuropathol. 2018. PMID: 29428972 Review.
-
The glymphatic pathway in neurological disorders.Lancet Neurol. 2018 Nov;17(11):1016-1024. doi: 10.1016/S1474-4422(18)30318-1. Lancet Neurol. 2018. PMID: 30353860 Free PMC article. Review.
-
The impact of neurovascular, blood-brain barrier, and glymphatic dysfunction in neurodegenerative and metabolic diseases.Int Rev Neurobiol. 2020;154:413-436. doi: 10.1016/bs.irn.2020.02.006. Epub 2020 Jul 14. Int Rev Neurobiol. 2020. PMID: 32739013 Review.
-
The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future.Annu Rev Pathol. 2018 Jan 24;13:379-394. doi: 10.1146/annurev-pathol-051217-111018. Annu Rev Pathol. 2018. PMID: 29195051 Free PMC article. Review.
Cited by
-
Potential of Multiscale Astrocyte Imaging for Revealing Mechanisms Underlying Neurodevelopmental Disorders.Int J Mol Sci. 2021 Sep 24;22(19):10312. doi: 10.3390/ijms221910312. Int J Mol Sci. 2021. PMID: 34638653 Free PMC article. Review.
-
Glymphatic system dysfunction in cerebral infarction: advances and perspectives based on DTI-derived ALPS measures.Am J Transl Res. 2025 Mar 15;17(3):1630-1642. doi: 10.62347/OQRE2088. eCollection 2025. Am J Transl Res. 2025. PMID: 40226042 Free PMC article. Review.
-
Brain Fluid Channels for Metabolite Removal.Physiol Res. 2022 Apr 30;71(2):199-208. doi: 10.33549/physiolres.934802. Epub 2022 Mar 28. Physiol Res. 2022. PMID: 35344669 Free PMC article. Review.
-
How Organ-on-a-Chip Technology Can Assist in Studying the Role of the Glymphatic System in Neurodegenerative Diseases.Int J Mol Sci. 2023 Jan 21;24(3):2171. doi: 10.3390/ijms24032171. Int J Mol Sci. 2023. PMID: 36768495 Free PMC article. Review.
-
Targeting autophagy in astrocytes: a potential for neurodegenerative disease intervention.Front Cell Neurosci. 2025 Apr 28;19:1584767. doi: 10.3389/fncel.2025.1584767. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40357169 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

