Deletion of Orphan G Protein-Coupled Receptor GPR37L1 in Mice Alters Cardiovascular Homeostasis in a Sex-Specific Manner
- PMID: 33633567
- PMCID: PMC7901490
- DOI: 10.3389/fphar.2020.600266
Deletion of Orphan G Protein-Coupled Receptor GPR37L1 in Mice Alters Cardiovascular Homeostasis in a Sex-Specific Manner
Abstract
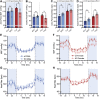
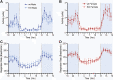
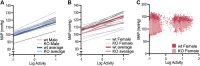
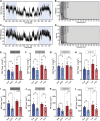
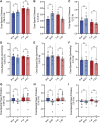
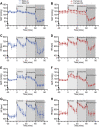
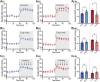
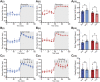
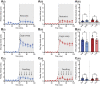
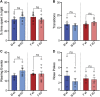
GPR37L1 is a family A orphan G protein-coupled receptor (GPCR) with a putative role in blood pressure regulation and cardioprotection. In mice, genetic ablation of Gpr37l1 causes sex-dependent effects; female mice lacking Gpr37l1 (GPR37L1-/-) have a modest but significant elevation in blood pressure, while male GPR37L1-/- mice are more susceptible to cardiovascular dysfunction following angiotensin II-induced hypertension. Given that this receptor is highly expressed in the brain, we hypothesize that the cardiovascular phenotype of GPR37L1-/- mice is due to changes in autonomic regulation of blood pressure and heart rate. To investigate this, radiotelemetry was employed to characterize baseline cardiovascular variables in GPR37L1-/- mice of both sexes compared to wildtype controls, followed by power spectral analysis to quantify short-term fluctuations in blood pressure and heart rate attributable to alterations in autonomic homeostatic mechanisms. Additionally, pharmacological ganglionic blockade was performed to determine vasomotor tone, and environmental stress tests were used to assess whether cardiovascular reactivity was altered in GPR37L1-/- mice. We observed that mean arterial pressure was significantly lower in female GPR37L1-/- mice compared to wildtype counterparts, but was unchanged in male GPR37L1-/- mice. GPR37L1-/- genotype had a statistically significant positive chronotropic effect on heart rate across both sexes when analyzed by two-way ANOVA. Power spectral analysis of these data revealed a reduction in power in the heart rate spectrum between 0.5 and 3 Hz in female GPR37L1-/- mice during the diurnal active period, which indicates that GPR37L1-/- mice may have impaired cardiac vagal drive. GPR37L1-/- mice of both sexes also exhibited attenuated depressor responses to ganglionic blockade with pentolinium, indicating that GPR37L1 is involved in maintaining sympathetic vasomotor tone. Interestingly, when these mice were subjected to aversive and appetitive behavioral stressors, the female GPR37L1-/- mice exhibited an attenuation of cardiovascular reactivity to aversive, but not appetitive, environmental stimuli. Together, these results suggest that loss of GPR37L1 affects autonomic maintenance of blood pressure, giving rise to sex-specific cardiovascular changes in GPR37L1-/- mice.
Keywords: G protein-coupled receptor; blood pressure; heart rate variability; hypertension; radiotelemetry; sex differences.
Copyright © 2021 Mouat, Jackson, Coleman, Paterson, Graham, Head and Smith.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1.Cells. 2022 Jun 1;11(11):1814. doi: 10.3390/cells11111814. Cells. 2022. PMID: 35681509 Free PMC article.
-
Orphan receptor GPR37L1 contributes to the sexual dimorphism of central cardiovascular control.Biol Sex Differ. 2018 Apr 6;9(1):14. doi: 10.1186/s13293-018-0173-y. Biol Sex Differ. 2018. PMID: 29625592 Free PMC article.
-
Involvement of GPR37L1 in murine blood pressure regulation and human cardiac disease pathophysiology.Am J Physiol Heart Circ Physiol. 2021 Oct 1;321(4):H807-H817. doi: 10.1152/ajpheart.00198.2021. Epub 2021 Sep 17. Am J Physiol Heart Circ Physiol. 2021. PMID: 34533400
-
Drug Discovery Opportunities at the Endothelin B Receptor-Related Orphan G Protein-Coupled Receptors, GPR37 and GPR37L1.Front Pharmacol. 2015 Nov 17;6:275. doi: 10.3389/fphar.2015.00275. eCollection 2015. Front Pharmacol. 2015. PMID: 26635605 Free PMC article. Review.
-
Brain angiotensin AT1 receptors as specific regulators of cardiovascular reactivity to acute psychoemotional stress.Clin Exp Pharmacol Physiol. 2011 Feb;38(2):126-35. doi: 10.1111/j.1440-1681.2010.05469.x. Clin Exp Pharmacol Physiol. 2011. PMID: 21143493 Review.
Cited by
-
Metabolic Profiling of Mice with Deletion of the Orphan G Protein-Coupled Receptor, GPR37L1.Cells. 2022 Jun 1;11(11):1814. doi: 10.3390/cells11111814. Cells. 2022. PMID: 35681509 Free PMC article.
-
Role of G-protein coupled receptors in cardiovascular diseases.Front Cardiovasc Med. 2023 Jun 5;10:1130312. doi: 10.3389/fcvm.2023.1130312. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37342437 Free PMC article. Review.
-
Rare GPR37L1 Variants Reveal Potential Association between GPR37L1 and Disorders of Anxiety and Migraine.J Neurosci. 2024 May 8;44(19):e1226232024. doi: 10.1523/JNEUROSCI.1226-23.2024. J Neurosci. 2024. PMID: 38569927 Free PMC article.
-
Impaired Aversive Memory Formation in GPR37L1KO Mice.Int J Mol Sci. 2022 Nov 18;23(22):14290. doi: 10.3390/ijms232214290. Int J Mol Sci. 2022. PMID: 36430766 Free PMC article.
-
G Protein-Coupled Receptor 37L1 Modulates Epigenetic Changes in Human Renal Proximal Tubule Cells.Int J Mol Sci. 2022 Nov 21;23(22):14456. doi: 10.3390/ijms232214456. Int J Mol Sci. 2022. PMID: 36430934 Free PMC article.
References
-
- Åstrand A., Bohlooly-Y M., Larsdotter S., Mahlapuu M., Andersén H., Tornell J., et al. (2004). Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R749–R758. 10.1152/ajpregu.00134.2004 - DOI - PubMed
-
- Baudrie V., Laude D., Elghozi J. L. (2007). Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R904–R912. 10.1152/ajpregu.00488.2006 - DOI - PubMed
-
- Bruder-Nascimento T., Ekeledo O. J., Anderson R., Le H. B., Belin de Chantemèle E. J. (2017). Long term high fat diet treatment: an appropriate approach to study the sex-specificity of the autonomic and cardiovascular responses to obesity in mice. Front. Physiol. 8, 32 10.3389/fphys.2017.00032 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

