Unilateral Nephrectomy Stimulates ERK and Is Associated With Enhanced Na Transport
- PMID: 33633581
- PMCID: PMC7901926
- DOI: 10.3389/fphys.2021.583453
Unilateral Nephrectomy Stimulates ERK and Is Associated With Enhanced Na Transport
Abstract
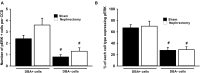
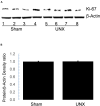
Nephron loss initiates compensatory hemodynamic and cellular effects on the remaining nephrons. Increases in single nephron glomerular filtration rate and tubular flow rate exert higher fluid shear stress (FSS) on tubules. In principal cell (PC) culture models FSS induces ERK, and ERK is implicated in the regulation of transepithelial sodium (Na) transport, as well as, proliferation. Thus, we hypothesize that high tubular flow and FSS mediate ERK activation in the cortical collecting duct (CCD) of solitary kidney which regulates amiloride sensitive Na transport and affects CCD cell number. Immunoblotting of whole kidney protein lysate was performed to determine phospho-ERK (pERK) expression. Next, sham and unilateral nephrectomized mice were stained with anti-pERK antibodies, and dolichos biflorus agglutinin (DBA) to identify PCs with pERK. Murine PCs (mpkCCD) were grown on semi-permeable supports under static, FSS, and FSS with U0126 (a MEK1/2 inhibitor) conditions to measure the effects of FSS and ERK inhibition on amiloride sensitive Na short circuit current (Isc). pERK abundance was greater in kidney lysate of unilateral vs. sham nephrectomies. The total number of cells in CCD and pERK positive PCs increased in nephrectomized mice (9.3 ± 0.4 vs. 6.1 ± 0.2 and 5.1 ± 0.5 vs. 3.6 ± 0.3 cell per CCD nephrectomy vs. sham, respectively, n > 6 per group, p < 0.05). However, Ki67, a marker of proliferation, did not differ by immunoblot or immunohistochemistry in nephrectomy samples at 1 month compared to sham. Next, amiloride sensitive Isc in static mpkCCD cells was 25.3 ± 1.7 μA/cm2 (n = 21), but after exposure to 24 h of FSS the Isc increased to 41.4 ± 2.8 μA/cm2 (n = 22; p < 0.01) and returned to 19.1 ± 2.1 μA/cm2 (n = 18, p < 0.01) upon treatment with U0126. Though FSS did not alter α- or γ-ENaC expression in mpkCCD cells, γ-ENaC was reduced in U0126 treated cells. In conclusion, pERK increases in whole kidney and, specifically, CCD cells after nephrectomy, but pERK was not associated with active proliferation at 1-month post-nephrectomy. In vitro studies suggest high tubular flow induces ERK dependent ENaC Na absorption and may play a critical role in Na balance post-nephrectomy.
Keywords: cortical collecting duct; electrophysiology; mitogen activated protein kinase; nephrectomy; sodium transport.
Copyright © 2021 Repetti, Majumder, De Oliveira, Meth, Yangchen, Sharma, Srivastava and Rohatgi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

