Activation of Orexin 1 Receptors in the Paraventricular Nucleus Contributes to the Development of Deoxycorticosterone Acetate-Salt Hypertension Through Regulation of Vasopressin
- PMID: 33633591
- PMCID: PMC7902066
- DOI: 10.3389/fphys.2021.641331
Activation of Orexin 1 Receptors in the Paraventricular Nucleus Contributes to the Development of Deoxycorticosterone Acetate-Salt Hypertension Through Regulation of Vasopressin
Abstract
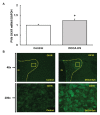
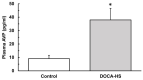
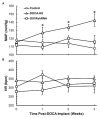
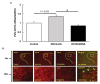
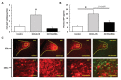
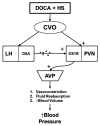
Salt-sensitivity is a major factor in the development of hypertension. The brain orexin system has been observed to play a role in numerous hypertensive animal models. However, orexin's role in the pathology of salt-sensitive hypertension (SSH) remains to be adequately explored. We assessed the impact of orexin hyperactivity in the pathogenesis of the deoxycorticosterone acetate (DOCA) - salt rat model, specifically through modulation of Arginine Vasopressin (AVP). Adult male rats were separated into three groups: vehicle control, DOCA-salt, and DOCA-salt+OX1R-shRNA. DOCA-salt rats received subcutaneous implantation of a 21-day release, 75 mg DOCA pellet in addition to saline drinking water (1% NaCl and 0.2% KCl). DOCA-salt+OX1R-shRNA rats received bilateral microinjection of AAV2-OX1R-shRNA into the paraventricular nucleus (PVN) to knockdown function of the Orexin 1-Receptor (OX1R) within that area. Following 2-week to allow full transgene expression, a DOCA pellet was administered in addition to saline drinking solution. Vehicle controls received sham DOCA implantation but were given normal water. During the 3-week DOCA-salt or sham treatment period, mean arterial pressure (MAP) and heart rate (HR) were monitored utilizing tail-cuff plethysmography. Following the 3-week period, rat brains were collected for either PCR mRNA analysis, as well as immunostaining. Plasma samples were collected and subjected to ELISA analysis. In line with our hypothesis, OX1R expression was elevated in the PVN of DOCA-salt treated rats when compared to controls. Furthermore, following chronic knockdown of OX1R, the hypertension development normally induced by DOCA-salt treatment was significantly diminished in the DOCA-salt+OX1R-shRNA group. A concurrent reduction in PVN OX1R and AVP mRNA was observed in concert with the reduced blood pressure following AAV2-OX1R-shRNA treatment. Similarly, plasma AVP concentrations appeared to be reduced in the DOCA-salt+OX1R-shRNA group when compared to DOCA-salt rats. These results indicate that orexin signaling, specifically through the OX1R in the PVN are critical for the onset and maintenance of hypertension in the DOCA-salt model. This relationship is mediated, at least in part, through orexin activation of AVP producing neurons, and the subsequent release of AVP into the periphery. Our results outline a promising mechanism underlying the development of SSH through interactions with the brain orexin system.
Keywords: blood pressure; deoxycorticosterone acetate; hypertension; orexin; paraventricular nucleus; vasopressin.
Copyright © 2021 Bigalke, Gao, Chen and Shan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Increased activity of the orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension.Am J Physiol Heart Circ Physiol. 2017 Dec 1;313(6):H1075-H1086. doi: 10.1152/ajpheart.00822.2016. Epub 2017 Jun 30. Am J Physiol Heart Circ Physiol. 2017. PMID: 28667055 Free PMC article.
-
Increased expression of magnocellular vasopressin mRNA in rats with deoxycorticosterone-acetate induced salt appetite.Neuroendocrinology. 1998 Aug;68(2):105-15. doi: 10.1159/000054356. Neuroendocrinology. 1998. PMID: 9705577
-
Changes of hypothalamic and plasma vasopressin in rats with deoxycorticosterone-acetate induced salt appetite.J Steroid Biochem Mol Biol. 1999 Jul-Aug;70(1-3):47-57. doi: 10.1016/s0960-0760(99)00094-1. J Steroid Biochem Mol Biol. 1999. PMID: 10529002
-
The Orexin System and Hypertension.Cell Mol Neurobiol. 2018 Mar;38(2):385-391. doi: 10.1007/s10571-017-0487-z. Epub 2017 Mar 27. Cell Mol Neurobiol. 2018. PMID: 28349223 Free PMC article. Review.
-
Orexin, orexin receptor antagonists and central cardiovascular control.Front Neurosci. 2013 Dec 30;7:257. doi: 10.3389/fnins.2013.00257. Front Neurosci. 2013. PMID: 24415993 Free PMC article. Review.
Cited by
-
Orexin, Sleep, Sympathetic Neural Activity, and Cardiovascular Function.Hypertension. 2022 Dec;79(12):2643-2655. doi: 10.1161/HYPERTENSIONAHA.122.19796. Epub 2022 Sep 23. Hypertension. 2022. PMID: 36148653 Free PMC article. Review.
-
The perspective of hypertension and salt intake in Chinese population.Front Public Health. 2023 Feb 17;11:1125608. doi: 10.3389/fpubh.2023.1125608. eCollection 2023. Front Public Health. 2023. PMID: 36875386 Free PMC article. Review.
-
Hypothalamic kinin B1 receptor mediates orexin system hyperactivity in neurogenic hypertension.Sci Rep. 2021 Oct 26;11(1):21050. doi: 10.1038/s41598-021-00522-0. Sci Rep. 2021. PMID: 34702886 Free PMC article.
-
Activation of Orexin System Stimulates CaMKII Expression.Front Physiol. 2021 Jul 1;12:698185. doi: 10.3389/fphys.2021.698185. eCollection 2021. Front Physiol. 2021. PMID: 34276418 Free PMC article.
-
Central nervous system mechanisms of salt-sensitive hypertension.Physiol Rev. 2025 Oct 1;105(4):1989-2032. doi: 10.1152/physrev.00035.2024. Epub 2025 May 2. Physiol Rev. 2025. PMID: 40315132 Review.
References
-
- Amin M. S., Wang H. W., Reza E., Whitman S. C., Tuana B. S., Leenen F. H. (2005). Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am. J. Phys. Regul. Integr. Comp. Phys. 289, R1787–R1797. 10.1152/ajpregu.00063.2005, PMID: - DOI - PubMed
-
- Backberg M., Hervieu G., Wilson S., Meister B. (2002). Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur. J. Neurosci. 15, 315–328. 10.1046/j.0953-816x.2001.01859.x, PMID: - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

