Translating New Synthetic Biology Advances for Biosensing Into the Earth and Environmental Sciences
- PMID: 33633695
- PMCID: PMC7901896
- DOI: 10.3389/fmicb.2020.618373
Translating New Synthetic Biology Advances for Biosensing Into the Earth and Environmental Sciences
Abstract
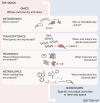
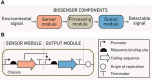
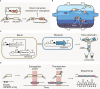
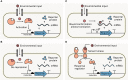
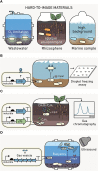
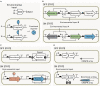
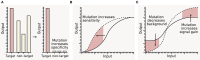
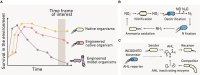
The rapid diversification of synthetic biology tools holds promise in making some classically hard-to-solve environmental problems tractable. Here we review longstanding problems in the Earth and environmental sciences that could be addressed using engineered microbes as micron-scale sensors (biosensors). Biosensors can offer new perspectives on open questions, including understanding microbial behaviors in heterogeneous matrices like soils, sediments, and wastewater systems, tracking cryptic element cycling in the Earth system, and establishing the dynamics of microbe-microbe, microbe-plant, and microbe-material interactions. Before these new tools can reach their potential, however, a suite of biological parts and microbial chassis appropriate for environmental conditions must be developed by the synthetic biology community. This includes diversifying sensing modules to obtain information relevant to environmental questions, creating output signals that allow dynamic reporting from hard-to-image environmental materials, and tuning these sensors so that they reliably function long enough to be useful for environmental studies. Finally, ethical questions related to the use of synthetic biosensors in environmental applications are discussed.
Keywords: biogeochemistry; biosensor; cell-free sensors; environmental microbiology; marine; soil; synthetic biology; wastewater.
Copyright © 2021 Del Valle, Fulk, Kalvapalle, Silberg, Masiello and Stadler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

