Progesterone Suppresses Neisseria gonorrhoeae-Induced Inflammation Through Inhibition of NLRP3 Inflammasome Pathway in THP-1 Cells and Murine Models
- PMID: 33633700
- PMCID: PMC7900005
- DOI: 10.3389/fmicb.2021.570093
Progesterone Suppresses Neisseria gonorrhoeae-Induced Inflammation Through Inhibition of NLRP3 Inflammasome Pathway in THP-1 Cells and Murine Models
Abstract
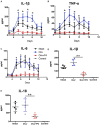
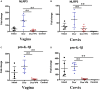
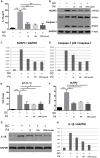
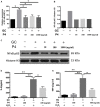
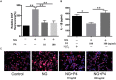
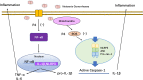
Asymptomatic/subclinical gonococcal infections in females continue to be prevalent within the general population, thus emerging as a global health problem. However, the reasons for these clinical manifestations are unknown. Our group had previously found out that in females, asymptomatic gonococcal infections correlate with higher serum progesterone (P4) levels and lower IL-1β levels in cervical secretions. We used murine infection model and THP-1 cells to determine whether P4 exerts anti-inflammatory effects on gonococcal infections. In the murine infection model, P4 (1 mg/day) inhibited the inflammatory effects induced by gonococcal infections which led to decreased neutrophil infiltration, reduced polymorphonuclear neutrophils (PMNs) numbers, IL-1β, TNF-α, and IL-6 levels in vaginal secretions. In addition, P4 down-regulated the mRNA and protein levels of NLRP3, associated with lower mRNA levels of pro-IL-1β, repressed caspase-1 activity in genital tissues and THP-1 cells. Moreover, P4 suppressed the phosphorylation levels of NF-κB and attenuated Neisseria gonorrhoeae (N. gonorrhoeae, gonococci or GC)-induced ROS generation. This is consistent with the two signals required for activation of the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome. In conclusion, our result shows that P4 suppresses the gonococci induced-inflammation, especially through the NLRP3 inflammasome pathway, and partially explains the pathogenesis of asymptomatic GC infection in women.
Keywords: NF-κB; NLRP3 inflammasome; Neisseria gonorrhoeae; inflammation; reactive oxygen species.
Copyright © 2021 Zhang, Zhang, Gan, Wei, Chai, A Aljaafreh, Liu, Duan, Jiang, Wang, He, Huang, Cai, Chen and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Honokiol Exhibits Anti-NLRP3 Inflammasome and Antimicrobial Properties in Neisseria gonorrhoeae-Infected Macrophages.J Inflamm Res. 2024 May 29;17:3499-3513. doi: 10.2147/JIR.S454221. eCollection 2024. J Inflamm Res. 2024. PMID: 38828053 Free PMC article.
-
HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production.J Hepatol. 2017 Apr;66(4):693-702. doi: 10.1016/j.jhep.2016.12.018. Epub 2016 Dec 24. J Hepatol. 2017. PMID: 28027970
-
Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye.Exp Eye Res. 2014 Aug;125:1-8. doi: 10.1016/j.exer.2014.05.001. Epub 2014 May 14. Exp Eye Res. 2014. PMID: 24836981
-
Koumine Suppresses IL-1β Secretion and Attenuates Inflammation Associated With Blocking ROS/NF-κB/NLRP3 Axis in Macrophages.Front Pharmacol. 2021 Jan 18;11:622074. doi: 10.3389/fphar.2020.622074. eCollection 2020. Front Pharmacol. 2021. PMID: 33542692 Free PMC article.
Cited by
-
Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation.Biomolecules. 2022 Sep 14;12(9):1299. doi: 10.3390/biom12091299. Biomolecules. 2022. PMID: 36139138 Free PMC article. Review.
-
Progesterone affects periodontitis in perimenopausal women and in an experimental rat model.J Dent Sci. 2025 Jan;20(1):452-461. doi: 10.1016/j.jds.2024.05.020. Epub 2024 May 24. J Dent Sci. 2025. PMID: 39873017 Free PMC article.
-
Exploring Candesartan, an angiotensin II receptor antagonist, as a novel inhibitor of NLRP3 inflammasome: alleviating inflammation in Neisseria gonorrhoeae infection.BMC Infect Dis. 2024 Nov 22;24(1):1338. doi: 10.1186/s12879-024-10208-3. BMC Infect Dis. 2024. PMID: 39578786 Free PMC article.
-
3D-printed bioceramic scaffolds for bone defect repair: bone aging and immune regulation.Front Bioeng Biotechnol. 2025 Mar 31;13:1557203. doi: 10.3389/fbioe.2025.1557203. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40242352 Free PMC article. Review.
-
The NLRP3 inflammasome in viral infection (Review).Mol Med Rep. 2023 Sep;28(3):160. doi: 10.3892/mmr.2023.13047. Epub 2023 Jul 7. Mol Med Rep. 2023. PMID: 37417336 Free PMC article. Review.
References
-
- Cavaillès P., Sergent V., Bisanz C., Papapietro O., Colacios C., Mas M., et al. (2006). The rat Toxo1 locus directs toxoplasmosis outcome and controls parasite proliferation and spreading by macrophage-dependent mechanisms. Proc. Natl. Acad. Sci. U.S.A. 103 744–749. 10.1073/pnas.0506643103 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

