Expression of NanoLuc Luciferase in Listeria innocua for Development of Biofilm Assay
- PMID: 33633716
- PMCID: PMC7901905
- DOI: 10.3389/fmicb.2021.636421
Expression of NanoLuc Luciferase in Listeria innocua for Development of Biofilm Assay
Abstract
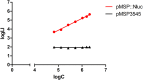
Studies of biofilm formation by bacteria are crucial for understanding bacterial resistance and for development of novel antibacterial strategies. We have developed a new bioluminescence biofilm assay for Listeria innocua, which is considered a non-pathogenic surrogate for Listeria monocytogenes. L. innocua was transformed with a plasmid for inducible expression of NanoLuc luciferase (Nluc). Concentration-dependent bioluminescence signals were obtained over a concentration range of more than three log units. This biofilm assay enables absolute quantification of bacterial cells, with the necessary validation. For biofilm detection and quantification, this "Nluc bioluminescence" method has sensitivity of 1.0 × 104 and 3.0 × 104 colony forming units (CFU)/mL, respectively, with a dynamic range of 1.0 × 104 to 5.0 × 107 CFU/mL. These are accompanied by good precision (coefficient of variation, <8%) and acceptable accuracy (relative error for most samples, <15%). This novel method was applied to assess temporal biofilm formation of L. innocua as a function of concentration of inoculant, in comparison with conventional plating and CFU counting, the crystal violet assay, and the resazurin fluorescence assay. Good correlation (r = 0.9684) of this Nluc bioluminescence assay was obtained with CFU counting. The limitations of this Nluc bioluminescence assay include genetic engineering of bacteria and relatively high cost, while the advantages include direct detection, absolute cell quantification, broad dynamic range, low time requirement, and high sensitivity. Nluc-based detection of L. innocua should therefore be considered as a viable alternative or a complement to existing methods.
Keywords: Listeria innocua; NanoLuc; biofilm assay; bioluminescence; luciferase.
Copyright © 2021 Berlec, Janež, Sterniša, Klančnik and Sabotič.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

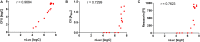
Similar articles
-
Listeria innocua Biofilm Assay Using NanoLuc Luciferase.Bio Protoc. 2022 Feb 5;12(3):e4308. doi: 10.21769/BioProtoc.4308. eCollection 2022 Feb 5. Bio Protoc. 2022. PMID: 35284607 Free PMC article.
-
Campylobacter jejuni Biofilm Assessment by NanoLuc Luciferase Assay.Bio Protoc. 2025 Feb 20;15(4):e5192. doi: 10.21769/BioProtoc.5192. eCollection 2025 Feb 20. Bio Protoc. 2025. PMID: 40028012 Free PMC article.
-
Engineered Reporter Phages for Rapid Bioluminescence-Based Detection and Differentiation of Viable Listeria Cells.Appl Environ Microbiol. 2020 May 19;86(11):e00442-20. doi: 10.1128/AEM.00442-20. Print 2020 May 19. Appl Environ Microbiol. 2020. PMID: 32245761 Free PMC article.
-
Detection and quantification of the iap gene of Listeria monocytogenes and Listeria innocua by a new real-time quantitative PCR assay.Res Microbiol. 2001 Jan-Feb;152(1):37-46. doi: 10.1016/s0923-2508(00)01166-9. Res Microbiol. 2001. PMID: 11281324
-
NanoLuc: A Small Luciferase Is Brightening Up the Field of Bioluminescence.Bioconjug Chem. 2016 May 18;27(5):1175-1187. doi: 10.1021/acs.bioconjchem.6b00112. Epub 2016 Apr 19. Bioconjug Chem. 2016. PMID: 27045664 Free PMC article. Review.
Cited by
-
Listeria innocua Biofilm Assay Using NanoLuc Luciferase.Bio Protoc. 2022 Feb 5;12(3):e4308. doi: 10.21769/BioProtoc.4308. eCollection 2022 Feb 5. Bio Protoc. 2022. PMID: 35284607 Free PMC article.
-
Listeria motility increases the efficiency of epithelial invasion during intestinal infection.PLoS Pathog. 2022 Dec 30;18(12):e1011028. doi: 10.1371/journal.ppat.1011028. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36584235 Free PMC article.
-
Holistic monitoring of Campylobacter jejuni biofilms with NanoLuc bioluminescence.Appl Microbiol Biotechnol. 2024 Dec 28;108(1):546. doi: 10.1007/s00253-024-13383-0. Appl Microbiol Biotechnol. 2024. PMID: 39731621 Free PMC article.
-
Modified vaginal lactobacilli expressing fluorescent and luminescent proteins for more effective monitoring of their release from nanofibers, safety and cell adhesion.Microb Cell Fact. 2024 Dec 19;23(1):333. doi: 10.1186/s12934-024-02612-w. Microb Cell Fact. 2024. PMID: 39696572 Free PMC article.
-
Comparative Analysis of NanoLuc Luciferase and Alkaline Phosphatase Luminescence Reporter Systems for Phage-Based Detection of Bacteria.Bioengineering (Basel). 2022 Sep 16;9(9):479. doi: 10.3390/bioengineering9090479. Bioengineering (Basel). 2022. PMID: 36135024 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

