Interferon α Enhances B Cell Activation Associated With FOXM1 Induction: Potential Novel Therapeutic Strategy for Targeting the Plasmablasts of Systemic Lupus Erythematosus
- PMID: 33633721
- PMCID: PMC7902015
- DOI: 10.3389/fimmu.2020.498703
Interferon α Enhances B Cell Activation Associated With FOXM1 Induction: Potential Novel Therapeutic Strategy for Targeting the Plasmablasts of Systemic Lupus Erythematosus
Abstract
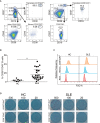
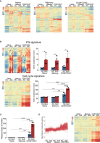
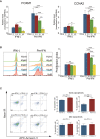
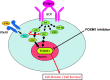
Systemic lupus erythematosus (SLE) is an autoimmune disease. It is characterized by the production of various pathogenic autoantibodies and is suggested to be triggered by increased type I interferon (IFN) signature. Previous studies have identified increased plasmablasts in the peripheral blood of SLE patients. The biological characteristics of SLE plasmablasts remain unknown, and few treatments that target SLE plasmablasts have been applied despite the unique cellular properties of plasmablasts compared with other B cell subsets and plasma cells. We conducted microarray analysis of naïve and memory B cells and plasmablasts (CD38+CD43+ B cells) that were freshly isolated from healthy controls and active SLE (n = 4, each) to clarify the unique biological properties of SLE plasmablasts. The results revealed that all B cell subsets of SLE expressed more type I IFN-stimulated genes. In addition, SLE plasmablasts upregulated the expression of cell cycle-related genes associated with higher FOXM1 and FOXM1-regulated gene expression levels than that in healthy controls. This suggests that a causative relationship exists between type I IFN priming and enhanced proliferative capacity through FOXM1. The effects of pretreatment of IFNα on B cell activation and FOXM1 inhibitor FDI-6 on B cell proliferation and survival were investigated. Pretreatment with IFNα promoted B cell activation after stimulation with anti-IgG/IgM antibody. Flow cytometry revealed that pretreatment with IFNα preferentially enhanced the Atk and p38 pathways after triggering B cell receptors. FDI-6 inhibited cell division and induced apoptosis in activated B cells. These effects were pronounced in activated B cells pretreated with interferon α. This study can provide better understanding of the pathogenic mechanism of interferon-stimulated genes on SLE B cells and an insight into the development of novel therapeutic strategies.
Keywords: B cell; FOXM1; interferon α; plasmablast; systemic lupus erythematosus.
Copyright © 2021 Akita, Yasaka, Shirai, Ishii, Harigae and Fujii.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Elevated STAT1 expression but not phosphorylation in lupus B cells correlates with disease activity and increased plasmablast susceptibility.Rheumatology (Oxford). 2020 Nov 1;59(11):3435-3442. doi: 10.1093/rheumatology/keaa187. Rheumatology (Oxford). 2020. PMID: 32357246
-
Effects of targeting the transcription factors Ikaros and Aiolos on B cell activation and differentiation in systemic lupus erythematosus.Lupus Sci Med. 2021 Mar;8(1):e000445. doi: 10.1136/lupus-2020-000445. Lupus Sci Med. 2021. PMID: 33727237 Free PMC article.
-
STAT3 phosphorylation mediates the stimulatory effects of interferon alpha on B cell differentiation and activation in SLE.Rheumatology (Oxford). 2020 Mar 1;59(3):668-677. doi: 10.1093/rheumatology/kez354. Rheumatology (Oxford). 2020. PMID: 31504941
-
Anti-interferon alpha treatment in SLE.Clin Immunol. 2013 Sep;148(3):303-12. doi: 10.1016/j.clim.2013.02.013. Epub 2013 Mar 1. Clin Immunol. 2013. PMID: 23566912 Review.
-
[BCMA and autoantibody-producing RP105 B cells; possible new targets of B cell therapy in systemic lupus erythematosus].Nihon Rinsho Meneki Gakkai Kaishi. 2012;35(1):38-45. doi: 10.2177/jsci.35.38. Nihon Rinsho Meneki Gakkai Kaishi. 2012. PMID: 22374441 Review. Japanese.
Cited by
-
Regulation of B Cell Responses in SLE by Three Classes of Interferons.Int J Mol Sci. 2021 Sep 28;22(19):10464. doi: 10.3390/ijms221910464. Int J Mol Sci. 2021. PMID: 34638804 Free PMC article. Review.
-
Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens.Vaccines (Basel). 2022 Apr 12;10(4):593. doi: 10.3390/vaccines10040593. Vaccines (Basel). 2022. PMID: 35455342 Free PMC article. Review.
-
Therapeutic Targeting of GSK3β-Regulated Nrf2 and NFκB Signaling Pathways by Salvianolic Acid A Ameliorates Peritoneal Fibrosis.Front Med (Lausanne). 2022 Mar 7;9:804899. doi: 10.3389/fmed.2022.804899. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35321474 Free PMC article.
-
FOXM1: Functional Roles of FOXM1 in Non-Malignant Diseases.Biomolecules. 2023 May 18;13(5):857. doi: 10.3390/biom13050857. Biomolecules. 2023. PMID: 37238726 Free PMC article. Review.
-
Systemic lupus erythematosus: latest insight into etiopathogenesis.Rheumatol Int. 2023 Aug;43(8):1381-1393. doi: 10.1007/s00296-023-05346-x. Epub 2023 May 24. Rheumatol Int. 2023. PMID: 37226016 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

