Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses
- PMID: 33633727
- PMCID: PMC7901974
- DOI: 10.3389/fimmu.2020.607333
Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses
Abstract
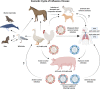
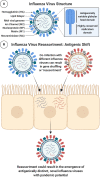
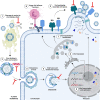
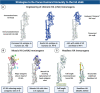
It is evident that the emergence of infectious diseases, which have the potential for spillover from animal reservoirs, pose an ongoing threat to global health. Zoonotic transmission events have increased in frequency in recent decades due to changes in human behavior, including increased international travel, the wildlife trade, deforestation, and the intensification of farming practices to meet demand for meat consumption. Influenza A viruses (IAV) possess a number of features which make them a pandemic threat and a major concern for human health. Their segmented genome and error-prone process of replication can lead to the emergence of novel reassortant viruses, for which the human population are immunologically naïve. In addition, the ability for IAVs to infect aquatic birds and domestic animals, as well as humans, increases the likelihood for reassortment and the subsequent emergence of novel viruses. Sporadic spillover events in the past few decades have resulted in human infections with highly pathogenic avian influenza (HPAI) viruses, with high mortality. The application of conventional vaccine platforms used for the prevention of seasonal influenza viruses, such as inactivated influenza vaccines (IIVs) or live-attenuated influenza vaccines (LAIVs), in the development of vaccines for HPAI viruses is fraught with challenges. These issues are associated with manufacturing under enhanced biosafety containment, and difficulties in propagating HPAI viruses in embryonated eggs, due to their propensity for lethality in eggs. Overcoming manufacturing hurdles through the use of safer backbones, such as low pathogenicity avian influenza viruses (LPAI), can also be a challenge if incompatible with master strain viruses. Non-replicating adenoviral (Ad) vectors offer a number of advantages for the development of vaccines against HPAI viruses. Their genome is stable and permits the insertion of HPAI virus antigens (Ag), which are expressed in vivo following vaccination. Therefore, their manufacture does not require enhanced biosafety facilities or procedures and is egg-independent. Importantly, Ad vaccines have an exemplary safety and immunogenicity profile in numerous human clinical trials, and can be thermostabilized for stockpiling and pandemic preparedness. This review will discuss the status of Ad-based vaccines designed to protect against avian influenza viruses with pandemic potential.
Keywords: adenoviral vector; adenovirus; avian influenza; highly pathogenic; highly pathogenic avian influenza; immunogenicity; influenza; vaccine.
Copyright © 2021 Kerstetter, Buckley, Bliss and Coughlan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

