Antibody Responses to Crude Gametocyte Extract Predict Plasmodium falciparum Gametocyte Carriage in Kenya
- PMID: 33633729
- PMCID: PMC7902058
- DOI: 10.3389/fimmu.2020.609474
Antibody Responses to Crude Gametocyte Extract Predict Plasmodium falciparum Gametocyte Carriage in Kenya
Abstract
Background: Malaria caused by Plasmodium falciparum remains a serious global public health challenge especially in Africa. Interventions that aim to reduce malaria transmission by targeting the gametocyte reservoir are key to malaria elimination and/or eradication. However, factors that are associated with gametocyte carriage have not been fully explored. Consequently, identifying predictors of the infectious reservoir is fundamental in the elimination campaign.
Methods: We cultured P. falciparum NF54 gametocytes (to stage V) and prepared crude gametocyte extract. Samples from a total of 687 participants (aged 6 months to 67 years) representing two cross-sectional study cohorts in Kilifi, Kenya were used to assess IgG antibody responses by ELISA. We also analyzed IgG antibody responses to the blood-stage antigen AMA1 as a marker of asexual parasite exposure. Gametocytemia and asexual parasitemia data quantified by microscopy and molecular detection (QT-NASBA) were used to determine the relationship with antibody responses, season, age, and transmission setting. Multivariable logistic regression models were used to study the association between antibody responses and gametocyte carriage. The predictive power of the models was tested using the receiver operating characteristic (ROC) curve.
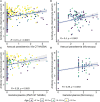
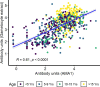
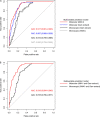
Results: Multivariable logistic regression analysis showed that IgG antibody response to crude gametocyte extract predicted both microscopic (OR=1.81 95% CI: 1.06-3.07, p=0.028) and molecular (OR=1.91, 95% CI: 1.11-3.29, p=0.019) P. falciparum gametocyte carriage. Antibody responses to AMA1 were also associated with both microscopic (OR=1.61 95% CI: 1.08-2.42, p=0.020) and molecular (OR=3.73 95% CI: 2.03-6.74, p<0.001) gametocytemia. ROC analysis showed that molecular (AUC=0.897, 95% CI: 0.868-0.926) and microscopic (AUC=0.812, 95% CI: 0.758-0.865) multivariable models adjusted for gametocyte extract showed very high predictive power. Molecular (AUC=0.917, 95% CI: 0.891-0.943) and microscopic (AUC=0.806, 95% CI: 0.755-0.858) multivariable models adjusted for AMA1 were equally highly predictive.
Conclusion: In our study, it appears that IgG responses to crude gametocyte extract are not an independent predictor of gametocyte carriage after adjusting for AMA1 responses but may predict gametocyte carriage as a proxy marker of exposure to parasites. Serological responses to AMA1 or to gametocyte extract may facilitate identification of individuals within populations who contribute to malaria transmission and support implementation of transmission-blocking interventions.
Keywords: Plasmodium falciparum; antibody response; gametocyte extract; gametocytemia; malaria transmission.
Copyright © 2021 Omondi, Muthui, Muasya, Orindi, Mwakubambanya, Bousema, Drakeley, Marsh, Bejon and Kapulu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- WHO World Malaria Report. Geneva: World Health Orginization, (2018).
-
- Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Tosukhowong T, Vannaphan S, et al. Decreased hemoglobin concentrations, hyperparasitemia, and severe malaria are associated with increased Plasmodium falciparum gametocyte carriage. J Parasitol (2002) 88(1):97–101. 10.1645/0022-3395(2002)088[0097:DHCHAS]2.0.CO;2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

