Amblyomma sculptum Salivary Protease Inhibitors as Potential Anti-Tick Vaccines
- PMID: 33633731
- PMCID: PMC7901972
- DOI: 10.3389/fimmu.2020.611104
Amblyomma sculptum Salivary Protease Inhibitors as Potential Anti-Tick Vaccines
Abstract
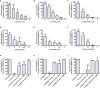
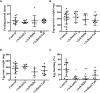
Amblyomma sculptum is the main tick associated with human bites in Brazil and the main vector of Rickettsia rickettsii, the causative agent of the most severe form of Brazilian spotted fever. Molecules produced in the salivary glands are directly related to feeding success and vector competence. In the present study, we identified sequences of A. sculptum salivary proteins that may be involved in hematophagy and selected three proteins that underwent functional characterization and evaluation as vaccine antigens. Among the three proteins selected, one contained a Kunitz_bovine pancreatic trypsin inhibitor domain (named AsKunitz) and the other two belonged to the 8.9 kDa and basic tail families of tick salivary proteins (named As8.9kDa and AsBasicTail). Expression of the messenger RNA (mRNA) encoding all three proteins was detected in the larvae, nymphs, and females at basal levels in unfed ticks and the expression levels increased after the start of feeding. Recombinant proteins rAs8.9kDa and rAsBasicTail inhibited the enzymatic activity of factor Xa, thrombin, and trypsin, whereas rAsKunitz inhibited only thrombin activity. All three recombinant proteins inhibited the hemolysis of both the classical and alternative pathways; this is the first description of tick members of the Kunitz and 8.9kDa families being inhibitors of the classical complement pathway. Mice immunization with recombinant proteins caused efficacies against A. sculptum females from 59.4% with rAsBasicTail immunization to more than 85% by immunization with rAsKunitz and rAs8.9kDa. The mortality of nymphs fed on immunized mice reached 70-100%. Therefore, all three proteins are potential antigens with the possibility of becoming a new tool in the control of A. sculptum.
Keywords: Amblyomma sculptum; antihemostatic; complement inhibitors; saliva; vaccine.
Copyright © 2021 Costa, Ribeiro, Melo-Junior, Gontijo, Sant’Anna, Pereira, Pessoa, Koerich, Oliveira, Valenzuela, Giunchetti, Fujiwara, Bartholomeu and Araujo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Gut membrane proteins as candidate antigens for immunization of mice against the tick Amblyomma sculptum.Vaccine. 2024 Aug 30;42(21):126141. doi: 10.1016/j.vaccine.2024.07.042. Epub 2024 Jul 20. Vaccine. 2024. PMID: 39033080 Free PMC article.
-
Target validation of highly conserved Amblyomma americanum tick saliva serine protease inhibitor 19.Ticks Tick Borne Dis. 2016 Apr;7(3):405-14. doi: 10.1016/j.ttbdis.2015.12.017. Epub 2015 Dec 29. Ticks Tick Borne Dis. 2016. PMID: 26746129 Free PMC article.
-
Analysis of the Salivary Gland Transcriptome of Unfed and Partially Fed Amblyomma sculptum Ticks and Descriptive Proteome of the Saliva.Front Cell Infect Microbiol. 2017 Nov 21;7:476. doi: 10.3389/fcimb.2017.00476. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29209593 Free PMC article.
-
Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development.J Vet Med Sci. 2001 Oct;63(10):1063-9. doi: 10.1292/jvms.63.1063. J Vet Med Sci. 2001. PMID: 11714020 Review.
-
Rhipicephalus (Boophilus) microplus embryo proteins as target for tick vaccine.Vet Immunol Immunopathol. 2012 Jul 15;148(1-2):149-56. doi: 10.1016/j.vetimm.2011.05.011. Epub 2011 May 7. Vet Immunol Immunopathol. 2012. PMID: 21620488 Review.
Cited by
-
Gut membrane proteins as candidate antigens for immunization of mice against the tick Amblyomma sculptum.Vaccine. 2024 Aug 30;42(21):126141. doi: 10.1016/j.vaccine.2024.07.042. Epub 2024 Jul 20. Vaccine. 2024. PMID: 39033080 Free PMC article.
-
Amblyostatin-1, the first salivary cystatin with host immunomodulatory and anti-inflammatory properties from the Neotropical tick Amblyomma sculptum, vector of Brazilian spotted fever.Front Immunol. 2025 Jul 17;16:1585703. doi: 10.3389/fimmu.2025.1585703. eCollection 2025. Front Immunol. 2025. PMID: 40746554 Free PMC article.
-
Resistance to Ticks and the Path to Anti-Tick and Transmission Blocking Vaccines.Vaccines (Basel). 2021 Jul 2;9(7):725. doi: 10.3390/vaccines9070725. Vaccines (Basel). 2021. PMID: 34358142 Free PMC article. Review.
-
Human Tick-Borne Diseases and Advances in Anti-Tick Vaccine Approaches: A Comprehensive Review.Vaccines (Basel). 2024 Jan 29;12(2):141. doi: 10.3390/vaccines12020141. Vaccines (Basel). 2024. PMID: 38400125 Free PMC article. Review.
-
Identification of Potential Amblyomma americanum Antigens After Vaccination with Tick Extracellular Vesicles in White-Tailed Deer.Vaccines (Basel). 2025 Mar 27;13(4):355. doi: 10.3390/vaccines13040355. Vaccines (Basel). 2025. PMID: 40333200 Free PMC article.
References
-
- Szabo MPJ, Martins TF, Barbieri ARM, Costa FB, Soares HS, Tolesano-Pascoli GV, et al. Ticks biting humans in the Brazilian savannah: Attachment sites and exposure risk in relation to species, life stage and season. Ticks Tick Borne Dis (2020) 11(2):101328. 10.1016/j.ttbdis.2019.101328 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

