Full Activation of Kinase Protein Kinase B by Phosphoinositide-Dependent Protein Kinase-1 and Mammalian Target of Rapamycin Complex 2 Is Required for Early Natural Killer Cell Development and Survival
- PMID: 33633735
- PMCID: PMC7901528
- DOI: 10.3389/fimmu.2020.617404
Full Activation of Kinase Protein Kinase B by Phosphoinositide-Dependent Protein Kinase-1 and Mammalian Target of Rapamycin Complex 2 Is Required for Early Natural Killer Cell Development and Survival
Abstract
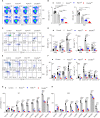
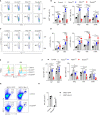
The role of PI3K-mTOR pathway in regulating NK cell development has been widely reported. However, it remains unclear whether NK cell development depends on the protein kinase B (PKB), which links PI3K and mTOR, perhaps due to the potential redundancy of PKB. PKB has two phosphorylation sites, threonine 308 (T308) and serine 473 (S473), which can be phosphorylated by phosphoinositide-dependent protein kinase-1 (PDK1) and mTORC2, respectively. In this study, we established a mouse model in which PKB was inactivated through the deletion of PDK1 and Rictor, a key component of mTORC2, respectively. We found that the single deletion of PDK1 or Rictor could lead to a significant defect in NK cell development, while combined deletion of PDK1 and Rictor severely hindered NK cell development at the early stage. Notably, ectopic expression of myristoylated PKB significantly rescued this defect. In terms of mechanism, in PDK1/Rictor-deficient NK cells, E4BP4, a transcription factor for NK cell development, was less expressed, and the exogenous supply of E4BP4 could alleviate the developmental defect of NK cell in these mice. Besides, overexpression of Bcl-2 also helped the survival of PDK1/Rictor-deficient NK cells, suggesting an anti-apoptotic role of PKB in NK cells. In summary, complete phosphorylation of PKB at T308 and S473 by PDK1 and mTORC2 is necessary for optimal NK cell development, and PKB regulates NK cell development by promoting E4BP4 expression and preventing cell apoptosis.
Keywords: development; mammalian target of rapamycin complex 2; natural killer (NK) cell; phosphoinositide-dependent protein kinase-1 (PDK1); protein kinase B (PKB); survival.
Copyright © 2021 He, Zhao, Quan, Hou, Yang and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

