Signaling Through Nucleic Acid Sensors and Their Roles in Inflammatory Diseases
- PMID: 33633744
- PMCID: PMC7902034
- DOI: 10.3389/fimmu.2020.625833
Signaling Through Nucleic Acid Sensors and Their Roles in Inflammatory Diseases
Abstract
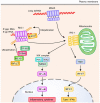
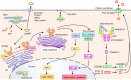
Recognition of pathogen-derived nucleic acids by pattern-recognition receptors (PRRs) is essential for eliciting antiviral immune responses by inducing the production of type I interferons (IFNs) and proinflammatory cytokines. Such responses are a prerequisite for mounting innate and pathogen-specific adaptive immune responses. However, host cells also use nucleic acids as carriers of genetic information, and the aberrant recognition of self-nucleic acids by PRRs is associated with the onset of autoimmune or autoinflammatory diseases. In this review, we describe the mechanisms of nucleic acid sensing by PRRs, including Toll-like receptors, RIG-I-like receptors, and DNA sensor molecules, and their signaling pathways as well as the disorders caused by uncontrolled or unnecessary activation of these PRRs.
Keywords: RIG-I-like receptor; Toll-like receptor; autoimmune disease; autoinflammatory disease; cGAMP synthase; nucleic acid sensing.
Copyright © 2021 Okude, Ori and Kawai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

