Unexpected rearrangements and a novel synthesis of 1,1-dichloro-1-alkenones from 1,1,1-trifluoroalkanones with aluminium trichloride
- PMID: 33633808
- PMCID: PMC7884876
- DOI: 10.3762/bjoc.17.36
Unexpected rearrangements and a novel synthesis of 1,1-dichloro-1-alkenones from 1,1,1-trifluoroalkanones with aluminium trichloride
Abstract
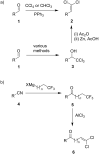
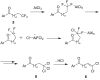
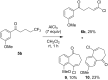
A novel reactivity of 1,1,1-trifluoroalkanones is reported, where the reaction with AlCl3 results in the formation of 1,1-dichloro-1-alkenones. The reaction scope was found to be broad, with various chain lengths and aryl substituents tolerated. For substrates containing an electron-rich aromatic ring, further reactions take place, resulting in bicyclic and/or rearrangement products.
Keywords: Friedel–Crafts alkylation; aluminium trichloride; dichloroalkenes; rearrangement; trifluoroalkanes.
Copyright © 2021, Lansbergen et al.
Figures





Similar articles
-
Synthesis of α-Aryl Oxindoles by Friedel-Crafts Alkylation of Arenes.J Org Chem. 2020 May 1;85(9):6172-6180. doi: 10.1021/acs.joc.0c00370. Epub 2020 Apr 20. J Org Chem. 2020. PMID: 32259447
-
Friedel-Crafts reactions of 2,2-difluorocyclopropanecarbonyl chloride: unexpected ring-opening chemistry.J Org Chem. 2011 May 6;76(9):3450-6. doi: 10.1021/jo200423y. Epub 2011 Apr 12. J Org Chem. 2011. PMID: 21452904
-
Facile Friedel-Crafts alkylation of arenes under solvent-free conditions.Org Biomol Chem. 2024 Mar 13;22(11):2187-2191. doi: 10.1039/d4ob00162a. Org Biomol Chem. 2024. PMID: 38391292
-
Aromatic C-H bond functionalization through organocatalyzed asymmetric intermolecular aza-Friedel-Crafts reaction: a recent update.Beilstein J Org Chem. 2023 Jun 28;19:956-981. doi: 10.3762/bjoc.19.72. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37404800 Free PMC article. Review.
-
Use of AlCl3 in Friedel Crafts arylation type reactions and beyond: an overview on the development of unique methodologies leading to N-heteroarenes.Org Biomol Chem. 2017 May 16;15(19):4042-4057. doi: 10.1039/c7ob00468k. Org Biomol Chem. 2017. PMID: 28443923 Review.
Cited by
-
Theoretical Investigation of para Amino-Dichloro Chalcone Isomers. Part II: A DFT Structure-Stability Study of the FMO and NLO Properties.ACS Omega. 2023 Jan 27;8(5):4937-4953. doi: 10.1021/acsomega.2c07148. eCollection 2023 Feb 7. ACS Omega. 2023. PMID: 36777615 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
