1,2,3-Triazoles as leaving groups: SNAr reactions of 2,6-bistriazolylpurines with O- and C-nucleophiles
- PMID: 33633809
- PMCID: PMC7884883
- DOI: 10.3762/bjoc.17.37
1,2,3-Triazoles as leaving groups: SNAr reactions of 2,6-bistriazolylpurines with O- and C-nucleophiles
Abstract
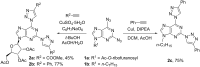
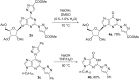
A new approach was designed for the synthesis of C6-substituted 2-triazolylpurine derivatives. A series of substituted products was obtained in SNAr reactions between 2,6-bistriazolylpurine derivatives and O- and C-nucleophiles under mild conditions. The products were isolated in yields up to 87%. The developed C-O and C-C bond forming reactions clearly show the ability of the 1,2,3-triazolyl ring at the C6 position of purine to act as leaving group.
Keywords: 2,6-bistriazolyl purines; nucleophilic aromatic substitution; purine nucleosides; triazoles.
Copyright © 2021, Cīrule et al.
Figures





References
-
- Dinesh S, Shikha G, Bhavana G, Nidhi S, Dileep S. J Pharm Sci Innovation. 2012;1:29–34.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
