Synthesis of trifluoromethyl ketones by nucleophilic trifluoromethylation of esters under a fluoroform/KHMDS/triglyme system
- PMID: 33633811
- PMCID: PMC7884878
- DOI: 10.3762/bjoc.17.39
Synthesis of trifluoromethyl ketones by nucleophilic trifluoromethylation of esters under a fluoroform/KHMDS/triglyme system
Abstract
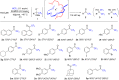
A straightforward method that enables the formation of biologically attractive trifluoromethyl ketones from readily available methyl esters using the potent greenhouse gas fluoroform (HCF3, HFC-23) was developed. The combination of fluoroform and KHMDS in triglyme at -40 °C was effective for this transformation, with good yields as high as 92%. Substrate scope of the trifluoromethylation procedure was explored for aromatic, aliphatic, and conjugated methyl esters. This study presents a straightforward trifluoromethylation process of various methyl esters that convert well to the corresponding trifluoromethyl ketones. The tolerance of various pharmacophores under the reaction conditions was also explored.
Keywords: HFC-23; fluoroform; greenhouse gas; trifluoromethyl ketones; trifluoromethylation.
Copyright © 2021, Fujihira et al.
Figures



Similar articles
-
Organocatalyzed Trifluoromethylation of Ketones and Sulfonyl Fluorides by Fluoroform under a Superbase System.ChemistryOpen. 2015 Oct;4(5):581-5. doi: 10.1002/open.201500160. Epub 2015 Aug 6. ChemistryOpen. 2015. PMID: 26491635 Free PMC article.
-
Gas/Liquid-Phase Micro-Flow Trifluoromethylation using Fluoroform: Trifluoromethylation of Aldehydes, Ketones, Chalcones, and N-Sulfinylimines.ChemistryOpen. 2019 Feb 5;8(4):406-410. doi: 10.1002/open.201800286. eCollection 2019 Apr. ChemistryOpen. 2019. PMID: 30976483 Free PMC article.
-
Direct nucleophilic trifluoromethylation of carbonyl compounds by potent greenhouse gas, fluoroform: Improving the reactivity of anionoid trifluoromethyl species in glymes.Sci Rep. 2018 Jul 31;8(1):11501. doi: 10.1038/s41598-018-29748-1. Sci Rep. 2018. PMID: 30065282 Free PMC article.
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
Regioselective C-H Trifluoromethylation and Its Related Reactions of (Hetero)aromatic Compounds.Chem Rec. 2023 Sep;23(9):e202300003. doi: 10.1002/tcr.202300003. Epub 2023 Mar 10. Chem Rec. 2023. PMID: 36899485 Review.
Cited by
-
Ex-situ generation and synthetic utilization of bare trifluoromethyl anion in flow via rapid biphasic mixing.Nat Commun. 2023 Mar 3;14(1):1231. doi: 10.1038/s41467-022-35611-9. Nat Commun. 2023. PMID: 36869027 Free PMC article.
-
Halo-perfluoroalkoxylation of gem-difluoroalkenes with short-lived alkali metal perfluoroalkoxides in triglyme.Chem Sci. 2024 May 22;15(25):9574-9581. doi: 10.1039/d4sc02084g. eCollection 2024 Jun 26. Chem Sci. 2024. PMID: 38939153 Free PMC article.
-
Copper(I)-free syntheses of [11C/18F]trifluoromethyl ketones from alkyl or aryl esters and [11C/18F]fluoroform.Chem Commun (Camb). 2024 Apr 23;60(34):4589-4592. doi: 10.1039/d4cc00465e. Chem Commun (Camb). 2024. PMID: 38577766 Free PMC article.
-
1,6-Nucleophilic Di- and Trifluoromethylation of para-Quinone Methides with Me3SiCF2H/Me3SiCF3 Facilitated by CsF/18-Crown-6.Molecules. 2024 Jun 19;29(12):2905. doi: 10.3390/molecules29122905. Molecules. 2024. PMID: 38930971 Free PMC article.
-
Repurposing of F-gases: challenges and opportunities in fluorine chemistry.Chem Soc Rev. 2022 Jun 20;51(12):4977-4995. doi: 10.1039/d1cs01072g. Chem Soc Rev. 2022. PMID: 35616085 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
