High Dosages of Equine Chorionic Gonadotropin Exert Adverse Effects on the Developmental Competence of IVF-Derived Mouse Embryos and Cause Oxidative Stress-Induced Aneuploidy
- PMID: 33634101
- PMCID: PMC7900142
- DOI: 10.3389/fcell.2020.609290
High Dosages of Equine Chorionic Gonadotropin Exert Adverse Effects on the Developmental Competence of IVF-Derived Mouse Embryos and Cause Oxidative Stress-Induced Aneuploidy
Abstract
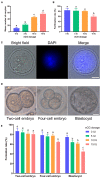
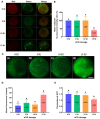
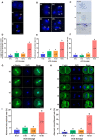
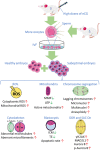
Gonadotropins play vital roles in the regulation of female reproductive ability and fertility. Our study aimed to determine the effects of superovulation induced by increasing doses of equine chorionic gonadotropin [eCG; also referred to as pregnant mare serum gonadotropin (PMSG)] on the developmental competence of mouse embryos and on aneuploidy formation during in vitro fertilization (IVF). eCG dose-dependently enhanced the oocyte yield from each mouse. Administration of 15 IU eCG significantly reduced the fertilization rate and the formation of four-cell embryos and blastocysts and increased the risk of chromosome aneuploidy. The IVF-derived blastocysts in the 15 IU eCG treatment group had the fewest total cells, inner cell mass (ICM) cells and trophectoderm (TE) cells. Moreover, more blastocysts and fewer apoptotic cells were observed in the 0, 5, and 10 IU eCG treatment groups than in the 15 IU eCG treatment group. We also investigated reactive oxygen species (ROS) levels and variations in several variables: mitochondrial membrane potential (MMP); active mitochondria; mitochondrial superoxide production; adenosine triphosphate (ATP) content; spindle structures; chromosome karyotypes; microfilament distribution; and the expression of Aurora B [an important component of the chromosomal passenger complex (CPC)], the spindle assembly checkpoint (SAC) protein mitotic arrest deficient 2 like 1 (MAD2L1), and the DNA damage response (DDR) protein γH2AX. Injection of 15 IU eCG increased ROS levels, rapidly reduced MMP, increased active mitochondria numbers and mitochondrial superoxide production, reduced ATP content, increased abnormal spindle formation rates, and induced abnormalities in chromosome number and microfilament distribution, suggesting that a high dose of eCG might alter developmental competence and exert negative effects on IVF-obtained mouse embryos. Additionally, the appearance of γH2AX and the significantly increased expression of Aurora B and MAD2L1 suggested that administration of relatively high doses of eCG caused Aurora B-mediated SAC activation triggered by ROS-induced DNA damage in early mouse IVF-derived embryos for self-correction of aneuploidy formation. These findings improve our understanding of the application of gonadotropins and provide a theoretical basis for gonadotropin treatment.
Keywords: Aurora B; DNA damage response; MAD2L1; aneuploidy; equine chorionic gonadotropin; reactive oxygen species; spindle assembly checkpoint.
Copyright © 2021 Lin, Li, Huang, Ru and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Aurora B prevents aneuploidy via MAD2 during the first mitotic cleavage in oxidatively damaged embryos.Cell Prolif. 2019 Sep;52(5):e12657. doi: 10.1111/cpr.12657. Epub 2019 Jul 1. Cell Prolif. 2019. PMID: 31264311 Free PMC article.
-
CHK1-CENP B/MAD2 is associated with mild oxidative damage-induced sex chromosome aneuploidy of male mouse embryos during in vitro fertilization.Free Radic Biol Med. 2019 Jun;137:181-193. doi: 10.1016/j.freeradbiomed.2019.04.037. Epub 2019 Apr 28. Free Radic Biol Med. 2019. PMID: 31042615
-
Development of a hamster superovulation program and adverse effects of gonadotropins on microfilament formation during oocyte development.Fertil Steril. 2005 Apr;83 Suppl 1:1264-74. doi: 10.1016/j.fertnstert.2004.09.039. Fertil Steril. 2005. PMID: 15831301
-
The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies.Hum Reprod Update. 2019 Jan 1;25(1):15-33. doi: 10.1093/humupd/dmy036. Hum Reprod Update. 2019. PMID: 30395265 Review.
-
Is the hypothesis of preimplantation genetic screening (PGS) still supportable? A review.J Ovarian Res. 2017 Mar 27;10(1):21. doi: 10.1186/s13048-017-0318-3. J Ovarian Res. 2017. PMID: 28347334 Free PMC article. Review.
Cited by
-
Maternal preconception stress produces sex-specific effects at the maternal:fetal interface to impact offspring development and phenotypic outcomes†.Biol Reprod. 2024 Feb 10;110(2):339-354. doi: 10.1093/biolre/ioad156. Biol Reprod. 2024. PMID: 37971364 Free PMC article.
-
Adverse effects of ovarian cryopreservation and auto-transplantation on ovarian grafts and quality of produced oocytes in a mouse model.Clin Sci (Lond). 2023 Oct 31;137(20):1577-1591. doi: 10.1042/CS20230483. Clin Sci (Lond). 2023. PMID: 37782233 Free PMC article.
-
Potential effects of assisted reproductive technology on telomere length and telomerase activity in human oocytes and early embryos.J Ovarian Res. 2023 Jul 4;16(1):130. doi: 10.1186/s13048-023-01211-4. J Ovarian Res. 2023. PMID: 37400833 Free PMC article. Review.
-
Biphasic oxygen tension promotes the formation of transferable blastocysts in patients without euploid embryos in previous monophasic oxygen cycles.Sci Rep. 2023 Mar 15;13(1):4330. doi: 10.1038/s41598-023-31472-4. Sci Rep. 2023. PMID: 36922540 Free PMC article.
-
The proteome, not the transcriptome, predicts that oocyte superovulation affects embryonic phenotypes in mice.Sci Rep. 2021 Dec 9;11(1):23731. doi: 10.1038/s41598-021-03054-9. Sci Rep. 2021. PMID: 34887460 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

