Involvement of the Actin Machinery in Programmed Cell Death
- PMID: 33634110
- PMCID: PMC7900405
- DOI: 10.3389/fcell.2020.634849
Involvement of the Actin Machinery in Programmed Cell Death
Abstract
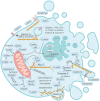
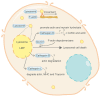
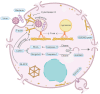
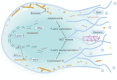
Programmed cell death (PCD) depicts a genetically encoded and an orderly mode of cellular mortality. When triggered by internal or external stimuli, cells initiate PCDs through evolutionary conserved regulatory mechanisms. Actin, as a multifunctional cytoskeleton protein that forms microfilament, its integrity and dynamics are essential for a variety of cellular processes (e.g., morphogenesis, membrane blebbing and intracellular transport). Decades of work have broadened our knowledge about different types of PCDs and their distinguished signaling pathways. However, an ever-increasing pool of evidences indicate that the delicate relationship between PCDs and the actin cytoskeleton is beginning to be elucidated. The purpose of this article is to review the current understanding of the relationships between different PCDs and the actin machinery (actin, actin-binding proteins and proteins involved in different actin signaling pathways), in the hope that this attempt can shed light on ensuing studies and the development of new therapeutic strategies.
Keywords: actin; actin machinery; actin-binding proteins; actin-modulating proteins; cytoskeleton; programmed cell death.
Copyright © 2021 Ren, Zhao, Cao and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Actin-binding proteins: the long road to understanding the dynamic landscape of cellular actin networks.Mol Biol Cell. 2016 Aug 15;27(16):2519-22. doi: 10.1091/mbc.E15-10-0728. Mol Biol Cell. 2016. PMID: 27528696 Free PMC article.
-
The yeast actin cytoskeleton: from cellular function to biochemical mechanism.Microbiol Mol Biol Rev. 2006 Sep;70(3):605-45. doi: 10.1128/MMBR.00013-06. Microbiol Mol Biol Rev. 2006. PMID: 16959963 Free PMC article. Review.
-
Programmed cell death in the cellular differentiation of microbial eukaryotes.Curr Opin Microbiol. 2012 Dec;15(6):646-52. doi: 10.1016/j.mib.2012.09.005. Epub 2012 Oct 17. Curr Opin Microbiol. 2012. PMID: 23083808 Review.
-
Microfilaments in cellular and developmental processes.Science. 1971 Jan 15;171(3967):135-43. doi: 10.1126/science.171.3967.135. Science. 1971. PMID: 5538822
-
Actin cytoskeleton: a signaling sensor in cell volume regulation.Cell Physiol Biochem. 2000;10(5-6):257-64. doi: 10.1159/000016366. Cell Physiol Biochem. 2000. PMID: 11125204 Review.
Cited by
-
Chemotherapeutic Potential of Chlorambucil-Platinum(IV) Prodrugs against Cisplatin-Resistant Colorectal Cancer Cells.Int J Mol Sci. 2024 Jul 28;25(15):8252. doi: 10.3390/ijms25158252. Int J Mol Sci. 2024. PMID: 39125821 Free PMC article.
-
Molecular and cellular level characterization of cytoskeletal mechanics using a quartz crystal microbalance.Cytoskeleton (Hoboken). 2023 May-Jun;80(5-6):100-111. doi: 10.1002/cm.21752. Epub 2023 Mar 24. Cytoskeleton (Hoboken). 2023. PMID: 36891731 Free PMC article. Review.
-
Disulfidptosis-associated LncRNA signature predicts prognosis and immune response in kidney renal clear cell carcinoma.Biol Direct. 2024 Aug 22;19(1):71. doi: 10.1186/s13062-024-00517-7. Biol Direct. 2024. PMID: 39175011 Free PMC article.
-
Anticancer Effect of PtIIPHENSS, PtII5MESS, PtII56MESS and Their Platinum(IV)-Dihydroxy Derivatives against Triple-Negative Breast Cancer and Cisplatin-Resistant Colorectal Cancer.Cancers (Basel). 2024 Jul 15;16(14):2544. doi: 10.3390/cancers16142544. Cancers (Basel). 2024. PMID: 39061185 Free PMC article.
-
Extracorporeal photopheresis as a promising strategy for the treatment of graft-versus-host disease after CAR T-cell therapy.Blood Adv. 2024 Jun 11;8(11):2675-2690. doi: 10.1182/bloodadvances.2023012463. Blood Adv. 2024. PMID: 38359409 Free PMC article.
References
-
- Alduaij W., Ivanov A., Honeychurch J., Cheadle E. J., Potluri S., Lim S. H., et al. . (2011). Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117, 4519–4529. 10.1182/blood-2010-07-296913 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

