Cell Populations Expressing Stemness-Associated Markers in Vascular Anomalies
- PMID: 33634164
- PMCID: PMC7900499
- DOI: 10.3389/fsurg.2020.610758
Cell Populations Expressing Stemness-Associated Markers in Vascular Anomalies
Abstract
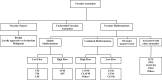
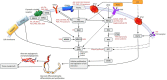
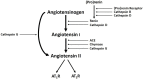
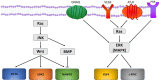
Treatment of vascular anomalies (VAs) is mostly empirical and, in many instances unsatisfactory, as the pathogeneses of these heterogeneous conditions remain largely unknown. There is emerging evidence of the presence of cell populations expressing stemness-associated markers within many types of vascular tumors and vascular malformations. The presence of these populations in VAs is supported, in part, by the observed clinical effect of the mTOR inhibitor, sirolimus, that regulates differentiation of embryonic stem cells (ESCs). The discovery of the central role of the renin-angiotensin system (RAS) in regulating stem cells in infantile hemangioma (IH) provides a plausible explanation for its spontaneous and accelerated involution induced by β-blockers and ACE inhibitors. Recent work on targeting IH stem cells by inhibiting the transcription factor SOX18 using the stereoisomer R(+) propranolol, independent of β-adrenergic blockade, opens up exciting opportunities for novel treatment of IH without the β-adrenergic blockade-related side effects. Gene mutations have been identified in several VAs, involving mainly the PI3K/AKT/mTOR and/or the Ras/RAF/MEK/ERK pathways. Existing cancer therapies that target these pathways engenders the exciting possibility of repurposing these agents for challenging VAs, with early results demonstrating clinical efficacy. However, there are several shortcomings with this approach, including the treatment cost, side effects, emergence of treatment resistance and unknown long-term effects in young patients. The presence of populations expressing stemness-associated markers, including transcription factors involved in the generation of induced pluripotent stem cells (iPSCs), in different types of VAs, suggests the possible role of stem cell pathways in their pathogenesis. Components of the RAS are expressed by cell populations expressing stemness-associated markers in different types of VAs. The gene mutations affecting the PI3K/AKT/mTOR and/or the Ras/RAF/MEK/ERK pathways interact with different components of the RAS, which may influence cell populations expressing stemness-associated markers within VAs. The potential of targeting these populations by manipulating the RAS using repurposed, low-cost and commonly available oral medications, warrants further investigation. This review presents the accumulating evidence demonstrating the presence of stemness-associated markers in VAs, their expression of the RAS, and their interaction with gene mutations affecting the PI3K/AKT/mTOR and/or the Ras/RAF/MEK/ERK pathways, in the pathogenesis of VAs.
Keywords: embryonic stem cells; gene mutations; induced pluripotent stem cells; renin-angiotensin system; stemness-associated markers; vascular anomalies; vascular malformation; vascular tumor.
Copyright © 2021 Kilmister, Hansen, Davis, Hall and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

