Impaired Left Atrial Performance Resulting From Age-Related Arial Fibrillation Is Associated With Increased Fibrosis Burden: Insights From a Clinical Study Combining With an in vivo Experiment
- PMID: 33634168
- PMCID: PMC7901954
- DOI: 10.3389/fcvm.2020.615065
Impaired Left Atrial Performance Resulting From Age-Related Arial Fibrillation Is Associated With Increased Fibrosis Burden: Insights From a Clinical Study Combining With an in vivo Experiment
Abstract
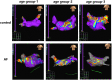
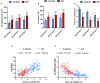
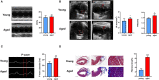
Background: Atrial fibrillation (AF) is increasingly considered an age-related degenerative disease, whose process is associated with the development of impaired left atrial (LA) performance. However, the subtle dynamic changes of LA performance in AF during aging have yet to be fully elucidated. Atrial fibrosis is a key substrate for the development of AF, but the progression of fibrosis during aging and its relationship with LA dysfunction need to be further explored. Methods: A total of 132 control individuals and 117 persistent AF patients were prospectively studied. Subjects were further stratified into three age groups (age group 1: younger than 65 years, age group 2: between 65 and 79 years old, and age group 3: older than 80 years). The two-dimensional speckle tracking imaging was carried out for analyzing the alterations in LA function underlying LA remodeling, whereas electroanatomic mapping was performed to investigate LA fibrosis burden. In animal study, aged mice and young mice served as research subjects. Echocardiography and histological staining were used to assess LA performance and fibrosis burden, respectively. Results: Echocardiography showed progressive increases in LA dimension and LA stiffness index, and progressive decreases in LA global longitudinal strain and LA strain rates with advancing age in both AF and control cohorts, which was more prominent in AF cohort. Electroanatomic mapping showed progressive decrease in mean LA voltage and progressive increases in LA surface area, low-voltage area %, and LA volume with advancing age, whereas more significant alterations were observed in AF patients. Moreover, left atrial global longitudinal strain was positively correlated with mean LA voltage, whereas LA stiffness index was negatively related to mean LA voltage. In animal experiment, increased LA size and pulmonary artery dimension as well as longer P-wave duration and more prominent LA fibrosis were found in aged mice. Conclusions: This study provides new evidence of subtle changes in structure and performance of left atrium and their association with atrial fibrosis in both AF and non-AF subjects during physiological aging. In addition, our study also provides normal values for LA structure and performance in both AF and non-AF conditions during aging. These measurements may provide an early marker for onset of AF and LA adverse remodeling.
Keywords: age; atrial fibrillation; echocardiography; fibrosis; left atrium performance.
Copyright © 2021 Lin, Chen, Li, Cai, Yuan, Wang, Zhang, Wei, Yan, Ma, Zheng, Wu, Li and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Relation of Echocardiographic Markers of Left Atrial Fibrosis to Atrial Fibrillation Burden.Am J Cardiol. 2018 Aug 15;122(4):584-591. doi: 10.1016/j.amjcard.2018.04.047. Epub 2018 Jun 4. Am J Cardiol. 2018. PMID: 30049466
-
The association between left atrial stiffness and low-voltage areas of left atrium in patients with atrial fibrillation.Heart Vessels. 2019 Nov;34(11):1830-1838. doi: 10.1007/s00380-019-01423-z. Epub 2019 May 2. Heart Vessels. 2019. PMID: 31049675
-
Mechanical and substrate abnormalities of the left atrium assessed by 3-dimensional speckle-tracking echocardiography and electroanatomic mapping system in patients with paroxysmal atrial fibrillation.Heart Rhythm. 2015 Mar;12(3):490-497. doi: 10.1016/j.hrthm.2014.12.007. Epub 2014 Dec 5. Heart Rhythm. 2015. PMID: 25485778
-
Early Echocardiographic Predictors for Atrial Fibrillation Propensity: The Left Atrium Oracle.Rev Cardiovasc Med. 2022 May 31;23(6):205. doi: 10.31083/j.rcm2306205. eCollection 2022 Jun. Rev Cardiovasc Med. 2022. PMID: 39077189 Free PMC article. Review.
-
From Left Atrial Dimension to Curved M-Mode Speckle-Tracking Images: Role of Echocardiography in Evaluating Patients with Atrial Fibrillation.Rev Cardiovasc Med. 2022 May 11;23(5):171. doi: 10.31083/j.rcm2305171. eCollection 2022 May. Rev Cardiovasc Med. 2022. PMID: 39077610 Free PMC article. Review.
Cited by
-
Analysis of the thickness characteristics of the left atrial posterior wall and its correlation with the low and no voltage areas of the left atrial posterior wall in patients with atrial fibrillation.J Cardiothorac Surg. 2024 Apr 6;19(1):187. doi: 10.1186/s13019-024-02658-2. J Cardiothorac Surg. 2024. PMID: 38582871 Free PMC article.
-
Time's imprint on the left atrium: aging and atrial myopathy.J Cardiovasc Aging. 2025 Jun;5(2):10.20517/jca.2024.23. doi: 10.20517/jca.2024.23. Epub 2025 Mar 20. J Cardiovasc Aging. 2025. PMID: 40896284
-
Echocardiographic Assessment of Cardiac Function in Mouse Models of Heart Disease.Int J Mol Sci. 2025 Jun 22;26(13):5995. doi: 10.3390/ijms26135995. Int J Mol Sci. 2025. PMID: 40649774 Free PMC article. Review.
-
How is Ambulatory Electrocardiogram Predictive of Stroke in Atrial Fibrillation Patients?Cardiol Res Pract. 2022 Oct 10;2022:7619669. doi: 10.1155/2022/7619669. eCollection 2022. Cardiol Res Pract. 2022. PMID: 36262211 Free PMC article.
-
Atrial fibrillation first? Investigating rhythm control in de novo high-grade functional mitral regurgitation and atrial fibrillation.Clin Res Cardiol. 2025 May 5. doi: 10.1007/s00392-025-02656-x. Online ahead of print. Clin Res Cardiol. 2025. PMID: 40323429
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

