Selected Uterine Immune Events Associated With the Establishment of Pregnancy in the Dog
- PMID: 33634180
- PMCID: PMC7900146
- DOI: 10.3389/fvets.2020.625921
Selected Uterine Immune Events Associated With the Establishment of Pregnancy in the Dog
Abstract
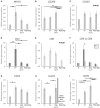
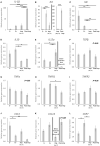
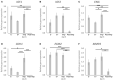
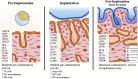
In the dog, implantation takes place at approximately 17 days of embryonal life and, while exposed to relatively high circulating progesterone concentrations, embryos presence is required for the formation of decidua. Furthermore, a balance between pro- and anti-inflammatory responses in conceptus-maternal communication is crucial for the onset of pregnancy. Strikingly, the understanding of such immune mechanisms in canine reproduction is still elusive. Here, canine uterine samples from pre-implantation (day 10-12, E+) and corresponding non-pregnant controls (E-), implantation (day 17, Imp) and post-implantation (day 18-25, Post-Imp) stages of pregnancy were used to investigate the expression and localization of several immune-related factors. The most important findings indicate increased availability of CD4, MHCII, NCR1, IDO1, AIF1, CD25, CCR7, and IL6 in response to embryo presence (E+), while FoxP3 and CCL3 were more abundant in E- samples. Implantation was characterized by upregulated levels of FoxP3, IL12a, ENG, and CDH1, whereas CD4, CCR7, IL8, and -10 were less represented. Following implantation, decreased transcript levels of TNFR1, MHCII, NCR1, TLR4, CD206, FoxP3, and IL12a were observed concomitantly with the highest expression of IL6 and IL1β. MHCII, CD86, CD206, CD163, TNFα, IDO1, and AIF1 were immunolocalized in macrophages, CD4 and Nkp46 in lymphocytes, and some signals of IDO1, AIF1, and TNF-receptors could also be identified in endothelial cells and/or uterine glands. Cumulatively, new insights regarding uterine immunity in the peri-implantation period are provided, with apparent moderated pro-inflammatory signals prevailing during pre-implantation, while implantation and early trophoblast invasion appear to be associated with immunomodulatory and rather anti-inflammatory conditions.
Keywords: dog (Canis lupus familiaris); early pregnancy; embryo-maternal communication; immune system; uterus.
Copyright © 2021 Tavares Pereira, Nowaczyk, Payan-Carreira, Miranda, Aslan, Kaya and Kowalewski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Utero-Placental Immune Milieu during Normal and Aglepristone-Induced Parturition in the Dog.Animals (Basel). 2021 Dec 19;11(12):3598. doi: 10.3390/ani11123598. Animals (Basel). 2021. PMID: 34944375 Free PMC article.
-
Canine conceptus-maternal communication during maintenance and termination of pregnancy, including the role of species-specific decidualization.Theriogenology. 2020 Jul 1;150:329-338. doi: 10.1016/j.theriogenology.2020.01.082. Epub 2020 Feb 19. Theriogenology. 2020. PMID: 32143817
-
Kisspeptin-10 and the G protein-coupled receptor 54 are differentially expressed in the canine pregnant uterus and trophoblast cells.Reprod Domest Anim. 2017 Apr;52 Suppl 2:123-129. doi: 10.1111/rda.12818. Epub 2016 Oct 23. Reprod Domest Anim. 2017. PMID: 27774662
-
Macrophages and migratory cells in endometrium relevant to implantation.Baillieres Clin Obstet Gynaecol. 1991 Mar;5(1):25-59. doi: 10.1016/s0950-3552(05)80069-0. Baillieres Clin Obstet Gynaecol. 1991. PMID: 1855342 Review.
-
Embryo development, hormonal requirements and maternal responses during canine pregnancy.J Reprod Fertil Suppl. 2001;57:169-79. J Reprod Fertil Suppl. 2001. PMID: 11787146 Review.
Cited by
-
The Canine Vaginal Flora: A Large-Cohort Retrospective Study.Vet Sci. 2024 Jan 27;11(2):55. doi: 10.3390/vetsci11020055. Vet Sci. 2024. PMID: 38393073 Free PMC article.
-
Transcriptomic profiling of canine decidualization and effects of antigestagens on decidualized dog uterine stromal cells.Sci Rep. 2022 Dec 19;12(1):21890. doi: 10.1038/s41598-022-24790-6. Sci Rep. 2022. PMID: 36535952 Free PMC article.
-
The canine vaginal microbiome during heat and fertility in healthy breeding dogs.PLoS One. 2025 Apr 28;20(4):e0321683. doi: 10.1371/journal.pone.0321683. eCollection 2025. PLoS One. 2025. PMID: 40293987 Free PMC article.
-
Utero-Placental Immune Milieu during Normal and Aglepristone-Induced Parturition in the Dog.Animals (Basel). 2021 Dec 19;11(12):3598. doi: 10.3390/ani11123598. Animals (Basel). 2021. PMID: 34944375 Free PMC article.
-
Canine Endotheliochorial Placenta: Morpho-Functional Aspects.Adv Anat Embryol Cell Biol. 2021;234:155-179. doi: 10.1007/978-3-030-77360-1_8. Adv Anat Embryol Cell Biol. 2021. PMID: 34694481
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

