How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19
- PMID: 33634345
- PMCID: PMC7906244
- DOI: 10.1007/s10654-021-00727-7
How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19
Abstract
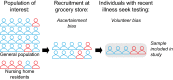
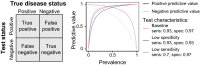
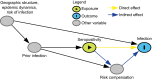
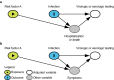
In response to the coronavirus disease (COVID-19) pandemic, public health scientists have produced a large and rapidly expanding body of literature that aims to answer critical questions, such as the proportion of the population in a geographic area that has been infected; the transmissibility of the virus and factors associated with high infectiousness or susceptibility to infection; which groups are the most at risk of infection, morbidity and mortality; and the degree to which antibodies confer protection to re-infection. Observational studies are subject to a number of different biases, including confounding, selection bias, and measurement error, that may threaten their validity or influence the interpretation of their results. To assist in the critical evaluation of a vast body of literature and contribute to future study design, we outline and propose solutions to biases that can occur across different categories of observational studies of COVID-19. We consider potential biases that could occur in five categories of studies: (1) cross-sectional seroprevalence, (2) longitudinal seroprotection, (3) risk factor studies to inform interventions, (4) studies to estimate the secondary attack rate, and (5) studies that use secondary attack rates to make inferences about infectiousness and susceptibility.
Keywords: COVID-19; Epidemiological biases; Measurement error; Misclassification; Observational data; Selection bias.
Conflict of interest statement
ML reports grants from NIH/NIGMS, during the conduct of the study; personal fees from Affinivax, personal fees from Merck, grants and personal fees from Pfizer, grants from PATH Vaccine Solutions, outside the submitted work.
Figures

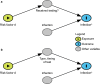

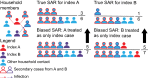
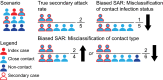

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

