Enriched Au nanoclusters with mesoporous silica nanoparticles for improved fluorescence/computed tomography dual-modal imaging
- PMID: 33634540
- PMCID: PMC8016642
- DOI: 10.1111/cpr.13008
Enriched Au nanoclusters with mesoporous silica nanoparticles for improved fluorescence/computed tomography dual-modal imaging
Abstract
Objectives: Au nanoclusters (AuNCs) have been used widely in fluorescence bio-imaging because of their good fluorescence, small particle size and non-cytotoxicity. AuNCs are also efficient in computed tomography (CT) imaging. Hence, a dual-modal imaging probe can be constructed without any complicated modification processes by exploiting the excellent performance of AuNCs. In the present study, AuNCs were enriched with mesoporous silica nanoparticles (MSNs) to obtain enhanced fluorescence/CT dual-modal imaging, which was capable of acquiring more imaging information for diseases compared with single-mode imaging.
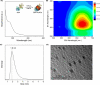
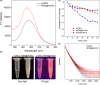
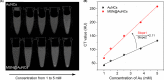
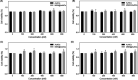
Materials and methods: Biocompatible bovine serum albumin (BSA)-capped AuNCs were prepared and loaded into amine-functionalized MSNs to form MSN@AuNCs. BSA-AuNCs, MSNs, and MSN@AuNCs were characterized by ultraviolet-visible (UV-vis) spectra, transmission electron microscopy (TEM), fluorescence spectra, and zeta potential. CT imaging was recorded using micro-CT scanning. Fluorescence imaging was measured using confocal laser scanning microscopy and flow cytometry.
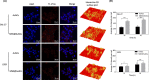
Results: The prepared AuNCs and MSNs possessed good properties as previously reported. The fluorescence intensity and CT value of the AuNCs were enhanced after being enriched with MSNs. The nanoparticles were both non-cytotoxic. Confocal laser scanning microscopy and flow cytometry indicated that MSN@AuNCs in CAL-27 cells showed improved fluorescence imaging compared with simple AuNCs at the same concentration.
Conclusions: The results revealed that the strategy of enriching AuNCs with MSNs can obtain highly sensitive fluorescence/CT dual-modal imaging, which indicated the potential of this nanoparticle in the diagnosis and treatment of disease.
Keywords: Au nanoclusters; computed tomography; dual-modal imaging; fluorescence; mesoporous silica nanoparticles.
© 2021 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest was declared of this article.
Figures


References
-
- Wang X, Xia J, Wang C, et al. Preparation of novel fluorescent nanocomposites based on au nanoclusters and their application in targeted detection of cancer cells. ACS Appl Mater Interfaces. 2017;9:44856‐44863. - PubMed
-
- Sugawara H, Suzuki S, Katada Y, et al. Comparison of full‐iodine conventional CT and half‐iodine virtual monochromatic imaging: advantages and disadvantages. Eur Radiol. 2019;29:1400‐1407. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

