A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY)
- PMID: 33634707
- PMCID: PMC8556578
- DOI: 10.1097/JU.0000000000001698
A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY)
Abstract
Purpose: Prostate specific membrane antigen-targeted positron emission tomography/computerized tomography has the potential to improve the detection and localization of prostate cancer. OSPREY was a prospective trial designed to determine the diagnostic performance of 18F-DCFPyL-positron emission tomography/computerized tomography for detecting sites of metastatic prostate cancer.
Materials and methods: Two patient populations underwent 18F-DCFPyL-positron emission tomography/computerized tomography. Cohort A enrolled men with high-risk prostate cancer undergoing radical prostatectomy with pelvic lymphadenectomy. Cohort B enrolled patients with suspected recurrent/metastatic prostate cancer on conventional imaging. Three blinded central readers evaluated the 18F-DCFPyL-positron emission tomography/computerized tomography. Diagnostic performance of 18F-DCFPyL-positron emission tomography/computerized tomography was based on imaging results compared to histopathology. In cohort A, detection of pelvic nodal disease (with specificity and sensitivity as co-primary end points) and of extrapelvic metastases were evaluated. In cohort B, sensitivity and positive predictive value for prostate cancer within biopsied lesions were evaluated.
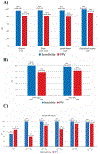
Results: A total of 385 patients were enrolled. In cohort A (252 evaluable patients), 18F-DCFPyL-positron emission tomography/computerized tomography had median specificity of 97.9% (95% CI: 94.5%-99.4%) and median sensitivity of 40.3% (28.1%-52.5%, not meeting prespecified end point) among 3 readers for pelvic nodal involvement; median positive predictive value and negative predictive value were 86.7% (69.7%-95.3%) and 83.2% (78.2%-88.1%), respectively. In cohort B (93 evaluable patients, median prostate specific antigen 11.3 ng/ml), median sensitivity and positive predictive value for extraprostatic lesions were 95.8% (87.8%-99.0%) and 81.9% (73.7%-90.2%), respectively.
Conclusions: The primary end point for specificity was met while the primary end point for sensitivity was not. The high positive predictive value observed in both cohorts indicates that 18F-DCFPyL-positive lesions are likely to represent disease, supporting the potential utility of 18F-DCFPyL-positron emission tomography/computerized tomography to stage men with high-risk prostate cancer for nodal or distant metastases, and reliably detect sites of disease in men with suspected metastatic prostate cancer.
Keywords: molecular imaging; neoplasm metastasis; neoplasm staging; prostatic neoplasms.
Conflict of interest statement
Financial interest and/or other relationship with Blue Earth, BK Medical, KOELIS, and OBP Medical and Perineologic.
Financial interest and/or other relationship with Insightec Francis Medical Norcal Lynx Group and Nutcracker Therapeutics.
Financial interest and/or other relationship with Astellas, Tersera and Janssen.
Financial interest and/or other relationship with AstraZeneca, Merck, BMS, Celgene, Prometheus, Arcus Biosciences, Pfizer, EMD Serono, Astellas and Seattle Genetics.
Financial interest and/or other relationship with Adient Medical Scientific and Verix Medical.
Financial interest and/or other relationship with Progenics Pharmaceuticals, Inc.
Financial interest and/or other relationship with Avid Radiopharmaceuticals, ImaginAb Inc., Blue Earth Diagnostics LLC, Capella Imaging LLC, Curium Pharma, General Electric Healthcare, Imaginab Inc., American College of Radiology, and American Medical Foundation for Peer Review and Education.
Financial interest and/or other relationship with ORIC Pharmaceuticals and Curium.
Figures



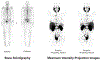
Comment in
-
Editorial Comment.J Urol. 2021 Jul;206(1):60-61. doi: 10.1097/JU.0000000000001698.01. Epub 2021 Apr 15. J Urol. 2021. PMID: 33853359 No abstract available.
References
-
- Shinohara K, Wheeler TM and Scardino PT: The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol 1989; 142: 76. - PubMed
-
- Hricak H, Dooms GC, Jeffrey RB et al. Prostatic carcinoma: staging by clinical assessment, CT, and MR imaging. Radiology 1987; 162: 331. - PubMed
-
- Scheidler J, Hricak H, Vigneron DB et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging—-clinicopathologic study. Radiology 1999; 213: 473. - PubMed
-
- Blomqvist L, Carlsson S, Gjertsson P et al. Limited evidence for the use of imaging to detect prostate cancer: a systematic review. Eur J Radiol 2014; 83: 1601. - PubMed
-
- Höovels AM, Heesakkers RAM, Adang EM et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008; 63: 387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

