Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1
Affiliations
- 1 Life Sciences Institute and Department of Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, MI, USA.
- 2 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt; Department of Experimental Oncology, IEO, European Institute of Oncology IRCCS, Milan, Italy.
- 3 Johannes Gutenberg University, Institute of Pharmaceutical and Biomedical Sciences, Department of Pharmaceutical Biology, Mainz, Germany.
- 4 Medical University of Graz, Division of Cardiology, Graz, Austria.
- 5 Pasteur institute of Iran, Department of Hepatitis and HIV, Tehran, Iran.
- 6 Department of Molecular Signal Processing, Leibniz Institute of Plant Biochemistry, Halle (Saale), Germany.
- 7 Hebrew University of Jerusalem, Department of Biochemistry and Food Science, Rehovot, Israel.
- 8 Danish Cancer Society Research Center, RNA and Autophagy Group, Copenhagen, Denmark.
- 9 University of Copenhagen, Biotech Research and Innovation Centre, Copenhagen, Denmark.
- 10 University of Tromsø - The Arctic University of Norway, Department of Medical Biology, Tromsø, Norway.
- 11 Hospital Universitario de Canarias, Research Unit; CIBERNED and Universidad de La Laguna, ITB, Santa Cruz de Tenerife, Spain.
- 12 University of California, Davis, Division of Rheumatology, Allergy and Clinical Immunology, School of Medicine, Sacramento, CA, USA.
- 13 University of Toronto, Department of Biochemistry, Toronto, Ontario, Canada.
- 14 Medical University Innsbruck, Department of Medicine I, Innsbruck, Austria.
- 15 University of Calabria, Department of Pharmacy, Health and Nutritional Sciences, Arcavacata di Rende (CS), Italy.
- 16 National Institutes of Health, National Eye institute, Protein Structure and Function Section, Bethesda, MD, USA.
- 17 Ben-Gurion University of the Negev, Faculty of Health Sciences, Department of Clinical Biochemistry and Pharmacology, Beer-Sheva, Israel.
- 18 University of Alabama at Birmingham, Division of Nephrology and Birmingham VA Medical Center, Birmingham, AL, USA.
- 19 Inflammation Research Center, San Diego, CA, USA.
- 20 University of Palermo, Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche (STEBICEF), Palermo, Italy.
- 21 Cell Death Research & Therapy (CDRT) Lab, Department of Cellular & Molecular Medicine, Center for Cancer Biology, VIB-KU Leuven, Leuven, Belgium.
- 22 Indian Institute of Technology, Ropar, India.
- 23 University of Dundee, School of Life Sciences, MRC Protein Phosphorylation and Ubiquitylation Unit, Dundee, UK.
- 24 University of Texas Medical Branch, Department of Pathology, Galveston, TX, USA.
- 25 Colby College, Department of Biology, Waterville, ME, USA.
- 26 University of Maryland School of Medicine, Departments of Otorhinolaryngology Head & Neck Surgery, Ophthalmology and Visual Science, Baltimore, MD, USA.
- 27 Universidad Mayor, Center for Integrative Biology, and Geroscience Center for Brain Health and Metabolism, Santiago, Chile.
- 28 Lund University, Department of Experimental Medical Science, Cell Death, Lysosomes and Artificial Intelligence Group, Lund, Sweden.
- 29 Nihon University, College of Bioresource Sciences, Fujisawa, Kanagawa, Japan.
- 30 Koç University School of Medicine, Department of Molecular Biology and Genetics and KUTTAM Research Center for Translational Medicine, Istanbul, Turkey.
- 31 University of Crete, School of Medicine, Laboratory of Clinical Microbiology and Microbial Pathogenesis, Voutes, Heraklion, Crete, Greece; Foundation for Research and Technology, Institute of Molecular Biology and Biotechnology (IMBB), Heraklion, Crete, Greece.
- 32 University of Crete, School of Medicine, Laboratory of Clinical Microbiology and Microbial Pathogenesis, Voutes, Heraklion, Crete, Greece.
- 33 The Institute of Cancer Research, Cancer Therapeutics Unit, Sutton, UK.
- 34 Pharmaceutical Sciences Department, College of Pharmacy, Gulf Medical University, Ajman, United Arab Emirates.
- 35 University of Sydney, Department of Pathology and Bosch Institute, Sydney, NSW, Australia.
- 36 Insight Institute of Neurosurgery & Neuroscience, Department of Research, Flint, MI, USA.
- 37 McGill University, Departments of Medicine and Oncology and Segal Cancer Centre, Montreal, Quebec, Canada.
- 38 Technical University Dresden, Center for Molecular and Cellular Bioengineering (CMCB), Biotechnology Center (BIOTEC), Department of Cellular Biochemistry, Dresden, Germany.
- 39 Universidad de Sevilla, Departamento de Psicología Experimental, Facultad de Psicología, Sevilla, Spain.
- 40 Sapienza University of Rome, Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Rome, Italy.
- 41 The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- 42 University of Rajshahi, Department of Pharmacy, Rajshahi, Bangladesh.
- 43 Department of Biological Sciences and Biotechnology, Faculty of Sciences, Islamic University of Gaza, Gaza 108, Palestine.
- 44 Department of Human Anatomy and Cell Science, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada.
- 45 University of Barcelona, Faculty of Pharmacy, Department of Biochemistry and Physiology, Barcelona, Spain.
- 46 Metabolism and Cancer Laboratory, Molecular Mechanisms and Experimental Therapy in Oncology Program (Oncobell), Institut d'Investigació Biomèdica de Bellvitge - IDIBELL, L'Hospitalet de Llobregat, Barcelona, Spain.
- 47 The Francis Crick Institute, Molecular Cell Biology of Autophagy, London, UK.
- 48 Cleveland Clinic, Department of Cancer Biology, Cleveland, OH, USA.
- 49 University of the Basque Country, Instituto Biofisika (UPV/EHU, CSIC), and Department of Biochemistry and Molecular Biology, Leioa, Spain.
- 50 Instituto de Investigaciones en Ingeniería Genética y Biología Molecular "Dr. Héctor N. Torres", Laboratorio de señalización y mecanismos adaptativos en Tripanosomátidos, Buenos Aires, Argentina; Universidad de Buenos Aires, Departamento de Fisiología, Biología Molecular y Celular, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina.
- 51 Laboratory of Host-Pathogen Dynamics, National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
- 52 The Wistar Institute, Philadelphia, PA, USA.
- 53 Buenos Aires University, Biochemistry and Pharmacy School, Deparment of Microbiology Immunology Biotechnology and Genetic, IDEHU, CABA, Argentina.
- 54 Universidade do Porto, i3S-Instituto de Investigação e Inovação em Saúde, Porto, Portugal; IPATIMUP - Institute of Molecular Pathology and Immunology of the University of Porto, Cancer Drug Resistance Group, Porto, Portugal; FFUP - Faculty of Pharmacy of the University of Porto, Department of Biological Sciences, Porto, Portugal.
- 55 Université Côte d'Azur, INSERM, CNRS, IPMC, team labeled Laboratory of Excellence (LABEX) Distalz, Valbonne, France.
- 56 Islamic University of Gaza, Department of Medical Laboratory Sciences, Faculty of Health Sciences, Gaza, Palestine.
- 57 University of Pavia, Department of Drug Sciences, Pharmacology Unit, Pavia, Italy.
- 58 University of Camerino, School of Biosciences and Veterinary Medicine, Camerino, Italy.
- 59 University of Porto, Faculty of Pharmacy, UCIBIO, REQUIMTE, Porto, Portugal.
- 60 Telethon Institute of Genetics and Medicine (TIGEM), Pozzuoli, Naples, Italy.
- 61 Ohio State University, Department of Microbial Infection and Immunity, and the Infectious Disease Research Institute, Columbus, OH, USA.
- 62 Save as Ravi Manjithaya.
- 63 University of California, San Francisco, San Francisco, CA, USA.
- 64 Aarhus University, Department of Molecular Biology and Genetics, Aarhus C, Denmark.
- 65 Departments of Pharmacology and Toxicology, and Neurology, The University of Alabama at Birmingham, Birmingham, AL, USA.
- 66 Federal University of São Paulo, Department of Medicine, Nephrology Division, Laboratory of Clinical and Experimental Immunology, São Paulo, SP, Brazil.
- 67 Universidade Nove de Julho (UNINOVE), Faculty of Pharmacy, São Paulo, SP, Brazil.
- 68 Cedars-Sinai Medical Center, Smidt Heart Institute, Los Angeles CA, USA.
- 69 University of Bologna, Department of Pharmacy and Biotechnology, Bologna, Italy.
- 70 City of Hope Comprehensive Cancer Center, Beckman Research Institute, Department of Diabetes Complications and Metabolism, Duarte, CA, USA.
- 71 University of Tennessee, Department of Chemical and Biomolecular Engineering, Knoxville, TN, USA.
- 72 Northeast Ohio Medical university, Department of Anatomy & Neurobiology, Rootstown, OH, USA.
- 73 iNOVA4Health Chronic Diseases Research Center (CEDOC), Faculdade de Ciências Médicas, Universidade Nova de Lisboa, Lisboa, Portugal.
- 74 Max Planck Institute for Biology of Ageing and CECAD, University of Cologne, Germany.
- 75 School of Biochemistry, Faculty of Life Sciences, University of Bristol, Bristol, UK.
- 76 University of Helsinki, Molecular and Integrative Biosciences Research Programme, Helsinki, Finland.
- 77 INSERM, U1231, Dijon, France.
- 78 University of Valencia, Faculty of Medicine, Department of Pharmacology, Valencia, Spain; CIBERehd (Centre for Networked Biomedical Research in Hepatic and Digestive Diseases), Valencia, Spain.
- 79 National Institute of Neuroscience, National Center of Neurology and Psychiatry, Department of Peripheral Nervous System Research, Kodaira, Tokyo, Japan.
- 80 Osaka University, Graduate School of Dentistry, Osaka, Japan.
- 81 Tokyo University of Pharmacy and Life Sciences, School of Life Sciences, Hachioji, Horinouchi, Tokyo, Japan.
- 82 Universidade Federal de Viçosa, Departamento de Biologia Vegetal, Viçosa, MG, Brazil.
- 83 The Jikei University School of Medicine, Division of Respiratory Disease, Departent of Internal Medicine, Tokyo, Japan.
- 84 Newcastle University, Biosciences Institute, Newcastle Upon Tyne, UK.
- 85 Instituto Cajal, Consejo Superior de Investigaciones Científicas (CSIC) and CIBERFES, Madrid, Spain.
- 86 University of Seville, Faculty of Pharmacy, Department of Physiology, Seville, Spain.
- 87 Albert Einstein College of Medicine, Department of Medicine, Bronx, NY, USA.
- 88 Howard University College of Medicine, Department of Anatomy, Washington D.C., USA.
- 89 Tohoku University, Graduate School fo Life Sciences, Sendai, Miyagi, Japan.
- 90 University of Michigan, Life Sciences Institute, Ann Arbor, MI, USA.
- 91 Imperial College London, MRC Centre for Molecular Bacteriolgy and Infection, Department of Microbiology, London, UK.
- 92 Toulouse University, CNRS, UPS, Center for Integrative Biology (CBI), Research Center on Animal Cognition (CRCA), Toulouse, France.
- 93 Iowa State University, Department of Genetics, Development and Cell Biology, Roy J. Carver Co-Laboratory, Ames, IA, USA.
- 94 Universidad Nacional de Cordoba, Laboratorio de Oncohematología, Hospital Nacional de Clínicas, CONICET, Córdoba, Argentina.
- 95 York College/The City University of New York, Department of Biology, Jamaica, NY, USA.
- 96 Translational Genomics Group, Incliva Health Research Institute, Burjasot, Valencia, Spain; Interdisciplinary Research Structure for Biotechnology and Biomedicine (ERI BIOTECMED), University of Valencia, Burjasot, Valencia, Spain.
- 97 University of Palermo, Department of Biological, Chemical and and Pharmaceutical Sciences and Technologies, Palermo, Italy.
- 98 Albert Einstein College of Medicine, Department of Molecular Pharmacology, Bronx, NY, USA.
- 99 Faculty of Engineering and Natural Sciences, Sabanci University, Orta Mahalle, Üniversite Caddesi No. 27, Orhanlı, Tuzla, 34956 Istanbul, Turkey; Sabanci University Nanotechnology Research and Application Center (SUNUM), Tuzla, 34956, Istanbul, Turkey.
- 100 Meir Medical Center, Translational Hemato-Oncology Laboratory, Kfar-Saba, Israel; Tel Aviv University, Department of Human Molecular Genetics and Biochemistry, Sackler School of Medicine, Tel Aviv, Israel.
- 101 The Institute of Genetics and Animal Biotechnology, Polish Academy of Sciences, Magdalenka, Poland; Ludwig Boltzmann Institute for Digital Health and Patient Safety, Medical University of Vienna, Vienna, Austria; Institute of Neurobiology, Bulgarian Academy of Sciences, Sofia, Bulgaria; Department of Pharmacognosy, University of Vienna, Vienna, Austria.
- 102 University of Pittsburgh Medical Center, Department of Critical Care Medicine, Department of Pediatrics, Safar Center for Resuscitation Research and the Children's Hospital of Pittsburgh, Pittsburgh, PA, USA.
- 103 University of Nice, Mediterranean Center for Molecular Medicine, Inserm U1065, Nice, France.
- 104 Imperial College London, Faculty of Medicine, London, UK.
- 105 Departments of Pharmacology and Microbiology, University of Maryland School of Medicine, Baltimore, MD, USA; Stanford University School of Medicine Stanford, CA, USA.
- 106 University of Turin, Department of Clinical and Biological Sciences, Turin, Italy.
- 107 University of Milan, Department of Health Sciences, Milano, Italy.
- 108 Universidad de Santiago de Chile, Faculty of Chemistry and Biology, Department of Biology, Santiago, Chile.
- 109 Fondazione Istituto di Ricerca Pediatrica Città della Speranza, Neuroblastoma Laboratory, Padua, Italy.
- 110 CNC - Center for Neuroscience and Cell Biology, Coimbra, IIIUC - Instituto de Investigação Interdisciplinar, University of Coimbra, Coimbra, Portugal.
- 111 The Hebrew University of Jerusalem, Alexander Silberman Institute of Life Sciences, Department of Plant and Environmental Sciences, Edmond Safra Campus, Jerusalem, Israel.
- 112 Tulane Health Sciences Center, Department of Pathology and Laboratory Sciences, New Orleans, LA, USA.
- 113 Melbourne Dementia Research Centre, The Florey Institute of Neuroscience & Mental Health, The University of Melbourne, Parkville, VIC, Australia.
- 114 University of Arkansas for Medical Sciences, Department of Geriatrics, Little Rock, AR, USA.
- 115 University of Malta, Department of Physiology & Biochemistry, Msida, Malta.
- 116 Kogakuin University, Research Institute for Science and Technology, Hachioji, Tokyo, Japan.
- 117 Albert Einstein College of Medicine, Departments of Molecular Pharmacology and Biochemistry, Bronx, NY, USA.
- 118 Eastern Michigan University, Department of Chemistry, Ypsilanti, MI, USA.
- 119 Hanyang University, College of Pharmacy, Ansan, Gyeonggido, Korea.
- 120 Yonsei University College of Medicine, Severance Biomedical Science Institute, Seoul, Korea.
- 121 University of Massachusetts Medical School, Department of Molecular, Cell and Cancer Biology, Worcester, MA, USA.
- 122 Konkuk University, Department of Bioscience and Biotechnology, Seoul, Korea.
- 123 Ajou University, College of Pharmacy and Research Institute of Pharmaceutical Science and Technology (RIPST), Suwon, Korea.
- 124 Seoul National University, Department of Biological Sciences, Seoul, South Korea.
- 125 University of Calabria, Department of Pharmacy, Health Science and Nutrition, Section of Preclinical and Translational Pharmacology, Cosenza, Italy.
- 126 Adam Mickiewicz University/Faculty of Biology, Institute of Experimental Biology, Department of General Botany, Poznan, Poland.
- 127 Iowa State University, Department of Genetics, Development, and Cell Biology, Ames, IA, USA.
- 128 Kunming University of Science and Technology, Medical School, Kunming, Yunnan, China.
- 129 National Jewish Health, Department of Academic Affairs, Denver, CO, USA.
- 130 University of Texas Health Science Center at San Antonio, Department of Cell Systems and Anatomy, San Antonio, TX, USA.
- 131 Jadavpur University, Department of Mathematics, Centre for Mathematical Biology and Ecology, Kolkata, India.
- 132 Karolinska Institute, Department of Cell and Molecular Biology, Stockholm, Sweden.
- 133 University of Genoa, Department of Earth, Environment and Life Sciences, Genoa, Italy.
- 134 University of Siena, Department of Life Sciences, Siena, Italy.
- 135 University of Urbino Carlo Bo, Department of Biomolecular Sciences, Urbino (PU), Italy.
- 136 Telethon Institute of Genetics and Medicine (TIGEM), Naples, Italy; Baylor College of Medicine, Houston, TX, USA.
- 137 University of Naples Federico II, Department of Translational Medical Sciences, Naples, Italy.
- 138 Institute for Research and Technology in Food and Agriculture (IRTA), Animal Breeding and Genetics Programme, Caldes de Montbui, Spain.
- 139 Max Planck Institute of Molecular Plant Physiology, Potsdam-Golm, Germany.
- 140 UCL Queen Square Institute of Neurology, Reta Lila Weston Institute, London, UK.
- 141 Middle East Technical University, Department of Biological Sciences, Ankara, Turkey.
- 142 University of Miami, Department of Surgery and Sylvester Comprehensive Cancer Center, Miami, FL, USA.
- 143 University of Miami, Department of Surgery, Miami, FL, USA.
- 144 Université Côte D'Azur, CNRS, Inserm, Institut de Biologie Valrose, Nice, France.
- 145 School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China.
- 146 Universidade de São Paulo. Department of Biochemistry, Institute of Chemistry, Sao Paulo, Brazil.
- 147 University of Bologna, Department of Biomedical and Neuromotor Sciences, Bologna, Italy.
- 148 University of Rome Tor Vergata, Department of Biology, Rome, Italy; Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione Santa Lucia, Laboratory of Cell Signaling, Rome, Italy.
- 149 Edinburgh Napier University, School of Applied Sciences, Edinburgh, UK.
- 150 University of Michigan, Department of Neurology, Ann Arbor, MI, USA.
- 151 Pulmonology Department, Hospital del Mar-IMIM, Pompeu Fabra University, CIBERES, Barcelona, Spain.
- 152 University of Limerick, Department of Biological Sciences, Limerick, Ireland; University of Limerick, Health Research Institute, Limerick, Ireland.
- 153 Danish Cancer Society Research Center, Copenhagen, Denmark.
- 154 Rice University, Department of Biosciences, Houston, TX, USA.
- 155 Columbia University, Department of Medicine, New York, NY, USA.
- 156 Jawaharlal Nehru Centre for Advanced Scientific Research, Molecular Biology and Genetics Unit, and Neuroscience Unit, Autophagy Laboratory, Jakkur, Bangalore, India.
- 157 ICAR-Indian Veterinary Research Institute, FMD Vaccine Research Laboratory, Bengaluru, India.
- 158 The University of Texas M.D. Anderson Cancer Center, Department of Experimental Therapeutics, Houston, TX, USA.
- 159 University of North Texas Health Science Center, Department of Microbiology, Immunology & Genetics, Fort Worth, TX, USA.
- 160 University of Louvain, Louvain Institute of Biomolecular Science and Technology (LIBST), Louvain-la-Neuve, Belgium.
- 161 Trinity College Dublin, School of Medicine, Trinity Translational Medicine Institute, Dublin, Ireland.
- 162 Université Paris-Saclay, INSERM UMR1195, Hôpital Le Kremlin Bicêtre, 80 rue du Général Leclerc, 94276 Kremlin-Bicêtre, France.
- 163 University of South Carolina Upstate, Spartanburg, SC, USA.
- 164 Institut National de la Santé et de la Recherche Médicale (INSERM), Sorbonne Université, Université de Paris, Centre de Recherche des Cordeliers, Paris, France.
- 165 Francis Crick Institute, London, UK; UCL, Royal Free Hospital, London, UK.
- 166 Université Paris-Saclay, Inserm U1185, Le Kremlin-Bicêtre, France.
- 167 Université de Pau et des Pays de l'Adour, E2S UPPA, INRAE, NUMEA, 64310 Saint-Pée-sur-Nivelle, France.
- 168 University of Sao Paulo, Institute of Biomedical Sciences, Sao Paulo, SP, Brazil.
- 169 Emory University School of Medicine, Division of Endocrinology, Metabolism and Lipids, Atlanta, GA, USA.
- 170 University of Pennsylvania, Perelman School of Medicine, PENN Center for Pulmonary Biology, Lung Epithelial Biology Laboratories, Philadelphia, PA, USA.
- 171 Mater Research Institute, University of Queensland, Brisbane Australia.
- 172 Ludwig-Maximilians-Universität München, Munich Cluster for Systems Neurology, München, Germany.
- 173 Hannover Medical School, Department for Clinical Immunology and Rheumatology, Hannover, Germany.
- 174 University of Rome "Tor Vergata", Department of Clinical Sciences and Translational Medicine, Rome, Italy.
- 175 Tufts University, USDA Human Nutrition Research Center on Aging, Laboratory for Nutrition and Vision Research, Boston, MA, USA.
- 176 Universidad Cardenal Herrera-CEU, CEU Universities, School of Health Sciences, Department of Biomedical Sciences, Moncada, Spain.
- 177 Bar-Ilan University, Azrieli Faculty of Medicine, Safed, Israel.
- 178 University Medical Center of the Johannes Gutenberg University, Institute of Pathobiochemistry, Mainz, Germany.
- 179 Boston University, Metcalf Science Center, Boston, MA, USA.
- 180 Sorbonne Université, CNRS UMR8226, Institut de Biologie Physico-Chimique, Laboratoire de Biologie Moléculaire et Cellulaire des Eucaryotes, Paris, France.
- 181 Italian Liver Foundation, Trieste, Italy.
- 182 University of Rome "Sapienza", Department of Clinical and Molecular Medicine, Laboratory affiliated to Istituto Pasteur Italia Fondazione Cenci Bolognetti, Rome, Italy.
- 183 Centro Nacional de Biotecnología-CNB-CSIC, Madrid, Spain.
- 184 Universidad Autónoma de Madrid, Departamento de Biología Molecular, Madrid, Spain.
- 185 University of São Paulo, Faculty of Pharmaceutical Sciences, Department of Pathophysiology, Sao Paulo, Brazil.
- 186 Universidad Mayor, Center for Integrative Biology, Santiago, Chile.
- 187 University of Oklahoma Health Sciences Center/Stephenson Cancer Center, Department of Obstetrics and Gynecology, Section of Gynecologic Oncology, Oklahoma City, OK, USA.
- 188 UMR 7242 "Biotechnology and cellular signaling", CNRS, Illkirch, France.
- 189 Washington University in Saint Louis, School of Medicine, Department of Psychiatry, Saint Louis MO, USA.
- 190 Universidad Francisco de Vitoria, Madrid, Spain.
- 191 Hopwood Centre for Neurobiology, Lifelong Health Theme, South Australian Health and Medical Research Institute, Adelaide, Australia.
- 192 University of Copenhagen, Department of Biology, Copenhagen, Denmark.
- 193 University of Lausanne Hospital and Lausanne University, Institute of Pathology, Department of Laboratory Medicine and Pathology, Lausanne, Switzerland.
- 194 Queen Mary University of London, Blizard Institute, Centre for Cell Biology and Cutaneous Research, London, UK.
- 195 University of Cologne and University Hospital Cologne, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany; Center for Molecular Medicine, Cologne, Germany.
- 196 University of Massachusetts Medical School, Department of Molecular, Cell and Cancer Biology (MCCB), Worcester, MA, USA.
- 197 Magna Graecia University, Department of Health Sciences, Catanzaro, Italy.
- 198 Université Paris Descartes, Institut Cochin, INSERM U1016, CNRS UMR8104, Paris, France.
- 199 CNRS, Université de Bordeaux, Laboratoire de Biogenèse Membranaire UMR5200, 33140 Villenave d'Ornon, Bordeaux, France; Université de Bordeaux, CNRS, Laboratoire de Biogenèse Membranaire UMR5200, Villenave d'Ornon, Bordeaux, France.
- 200 Université du Québec à Trois-Rivières, Département de Biologie Médicale, Trois-Rivières, Québec, Canada.
- 201 University of Michigan, Department of Ophthalmology and Visual Sciences, Ann Arbor, MI, USA.
- 202 University of Montpellier, CNRS/UMR5235 LPHI, Montpellier, France.
- 203 Bristol Medical School, University of Bristol, Bristol, UK.
- 204 Ghent University, VIB Center for Inflammation Research, Ghent, Belgium.
- 205 University of Oxford, Kennedy Institute of Rheumatology, Oxford, UK.
- 206 University of New Mexico, Department of Molecular Genetics and Microbiology, Department of Neurology, Albuquerque, NM, USA.
- 207 Comenius University, Department of Biochemistry, Bratislava, Slovakia.
- 208 University of Oklahoma Health Sciences Center, Department of Obstetrics and Gynecology, Oklahoma City, OK, USA.
- 209 Vanderbilt University Medical Center, Vanderbilt Eye Institute, Nashville, TN, USA.
- 210 Department of Biophysics, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- 211 Louisiana State University Health Sciences Center-Shreveport, Department of Pathology and Translational Pathobiology, Shreveport, LA, USA.
- 212 National Institute of Technology Rourkela, Department of Life Science, Cancer and Cell Death Laboratory, Rourkela, Odisha, India.
- 213 Michigan Technological University, Department of Chemistry, Houghton, MI, USA.
- 214 Dalian Medical University, College of Basic Medical Sciences, Dalian, China.
- 215 Garvan Institute of Medical Research, St Vincent's Medical School, Faculty of Medicine, UNSW, Sydney, Australia.
- 216 Eötvös Loránd University (ELTE), Department of Genetics, Budapest, Hungary.
- 217 Federal University of São Paulo, Department of Pharmacology, Paulista School of Medicine, São Paulo, Brazil.
- 218 University Hospital of North Norway, The Heart and Lung Clinic; University of Tromso-The Artic University of Norway, Department of Clinical Medicine, Tromso, Norway.
- 219 Norwegian University of Science and Technology, Department of Biomedical Laboratory Science, Trondheim, Norway.
- 220 University of York, Department of Biology, Heslington, UK.
- 221 University of Valencia, Faculty of Medicine, Department of Physiology, Valencia, Spain; CIBERehd, Valencia, Spain.
- 222 University of Lodz, Faculty of Biology and Environmental Protection, Lodz, Poland.
- 223 Linköping University, Department of Biomedical and Clinical Sciences, Faculty of Medicine and Health Sciences, Linköping, Sweden.
- 224 Karolinska Institutet, Department of Women's and Children's Health, Stockholm, Sweden AND Karolinska University Hospital, Pediatric Oncology, Stockholm, Sweden.
- 225 Indiana University School of Medicine, Department of Microbiology and Immunology, Indianapolis, IN, USA.
- 226 St. Jude Children's Research Hospital, Department of Immunology, Memphis, TN, USA.
- 227 University of Zagreb, School of Medicine, Croatian Institute for Brain Research, Zagreb, Croatia.
- 228 University of Pennsylvania, Department of Basic and Translational Sciences, Philadelphia, PA, USA.
- 229 Burnet Institute, Melbourne, Australia.
- 230 University College Cork, Department of Pharmacology and Therapeutics, County Cork, Ireland.
- 231 INMG, INSERM, CNRS, University of Lyon, Lyon, France.
- 232 University of Padova, Department of Molecular Medicine, Padova, Italy.
- 233 Institut National de la Santé et de la Recherche Médicale, Centre de Recherche des Cordeliers, Sorbonne Université, Université de Paris, Paris, France.
- 234 National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Neurosciences and Cellular and Structural Biology Division (NCSBD), Bethesda, MD, USA.
- 235 West Virginia University, Department of Surgery, Morgantown, WV, USA.
- 236 The Open University, School of Life, Health and Chemical Sciences, Faculty of Science, Technology, Engineering and Mathematics, Milton Keynes, UK.
- 237 University of Rome Tor Vergata, Department of Biology, Rome, Italy.
- 238 IRCCS Bambino Gesù Children's Hospital, Department of Pediatric Hemato-Oncology and Cell and Gene Therapy, Rome, Italy.
- 239 Albert Ludwigs University of Freiburg, Faculty of Medicine, Institute of Molecular Medicine and Cell Research, Freiburg, Germany.
- 240 University Children's Hospital Zurich, Children's Research Center and Department of Oncology, Zurich, Switzerland.
- 241 University of Texas M.D. Anderson Cancer Center, Department of Leukemia, Houston, TX, USA.
- 242 Case Western Reserve University School of Medicine, Department of Pediatrics, Cleveland, OH, USA; InterRayBio, LLC, Baltimore, MD, USA.
- 243 Washington State University, Department of Veterinary Microbiology and Pathology, College of Veterinary Medicine, Pullman, WA, USA.
- 244 University Santiago de Compostela, Department of Pharmacology, Veterinary Faculty, Lugo, Spain.
- 245 Baylor College of Medicine, Jan and Dan Duncan Neurological Research Institute and Department of Molecular and Human Genetics, Houston, TX, USA.
- 246 Université de Paris, PARCC, INSERM, Paris, France.
- 247 University of Alabama at Birmingham, Department of Ophthalmology and Visual Sciences, Birmingham, AL, USA.
- 248 Albert Einstein College of Medicine, Department of Developmental and Molecular Biology, Bronx, NY, USA.
- 249 Medical University of Graz, Gottfried Schatz Research Center for Cell Signaling, Metabolism and Aging, Molecular Biology and Biochemistry, Graz, Austria.
- 250 University Paris 13, UMR_S942 Inserm; University of Paris, Bobigny, France.
- 251 Centro de Investigaciones Biológicas Margarita Salas, CSIC, Madrid, Spain.
- 252 Swedish University of Agricultural Sciences and Linnean Center for Plant Biology, Uppsala BioCenter, Department of Molecular Sciences, Uppsala, Sweden.
- 253 Stanford University, Department of Chemical & Systems Biology, Stanford, CA, USA.
- 254 Imperial College London, Department of Life Sciences, London, UK.
- 255 The Connecticut Agricultural Experiment Station, Center for Vector Biology and Zoonotic Diseases, Department of Environmental Sciences, New Haven, CT, USA.
- 256 Goethe University, University Hospital, University Cancer Center Frankfurt (UCT), Department of Medicine, Hematology/Oncology, Frankfurt, Germany.
- 257 Danube Private University, Department of Medicine/Dental Medicine, Krems/Donau, Austria.
- 258 Georg-August-Universität Göttingen, Institute for Microbiology and Genetics, Department of Molecular Microbiology and Genetics, Göttingen, Germany.
- 259 Universidad de Chile, Instituto de Nutrición y Tecnología de los Alimentos (INTA), Santiago, Chile.
- 260 Université de Paris, Sorbonne Université, Centre de Recherche des Cordeliers, INSERM U1138, Paris, France.
- 261 University Côte d'Azur (UCA), Institute for Research on Cancer and Aging of Nice (IRCAN), Centre National de la Recherche Scientifique (CNRS) UMR7284, Institut National de la Santé et de la Recherche Médicale (INSERM) U1081, Nice, France.
- 262 Centre des Sciences du Goût et de l'Alimentation, AgroSup Dijon, CNRS, INRAE, University of Bourgogne Franche-Comté, Eye and Nutrition Research Group, Dijon, France.
- 263 National Autonomous University of Mexico, Faculty of Chemistry, Department of Biology, Mexico city, Mexico.
- 264 University of California San Francisco, Department of Medicine, Zuckerberg San Francisco General Hospital and TraumaCenter, San Francisco, CA, USA.
- 265 University of Copenhagen, Department of Biology, Denmark.
- 266 University of Pittsburgh, Department of Biological Sciences, Pittsburgh, PA, USA.
- 267 Washington University School of Medicine, Department of Medicine, Saint Louis, MO, USA.
- 268 Department of Human Genetics, Genentech, Inc., South San Francisco, CA, USA.
- 269 David Geffen School of Medicine at UCLA, Department of Neurology, Los Angeles, CA, USA.
- 270 University of Colorado, AMC, Department of Pharmacology, Aurora, CO, USA.
- 271 University of Minnesota, Hormel Institute, Austin, MN, USA.
- 272 University of Sao Paulo, School of Physical Education and Sport, Cellular and Molecular Exercise Physiology Laboratory, Sao Paulo, SP, Brazil.
- 273 Hospital for Sick Children, Cell Biology Program, Toronto, ON, Canada.
- 274 University of Insubria, Department of Biotechnology and Life Sciences, Varese, Italy.
- 275 Monash University, School of Biological Sciences, Melbourne, Victoria, Australia.
- 276 University of Salento, Dept of Biological and Environmental Sciences and Technologies, Lecce, Italy.
- 277 Institut Pasteur, Biologie des Bactéries Intracellulaires and CNRS UMR 3525, Paris, France.
- 278 University of Pittsburgh, Aging Institute, Department of Medicine, Pittsburgh, PA, USA.
- 279 University Duisburg-Essen, Essen University Hospital, Department of Gastroenterology and Hepatology, Essen, Germany; Department of Gastroenterology, Hepatology & Endocrinology, Hannover Medical School, Hannover, Germany.
- 280 Thomas Jefferson University, Department of Pathology, Anatomy, and Cell Biology, Philadelphia, PA, USA.
- 281 Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA.
- 282 University of Arizona, Department of Molecular and Cellular Biology, Tucson, AZ, USA.
- 283 University of Iowa, Iowa City, IA, USA.
- 284 Montana BioAg. Inc. Missoula, MT, USA.
- 285 Universidad de Chile, Facultad de Odontología, Autophagy Research Center (ARC), Santiago, Chile.
- 286 KU Leuven, Department of Cellular & Molecular Medicine, Campus Gasthuisberg, Leuven, Belgium.
- 287 University of Medicine and Pharmacy of Craiova, Department of Medical Genetics, Craiova, Romania.
- 288 King's College London, School of Cardiovascular Medicine & Sciences, The Rayne Institute, St Thomas' Hospital, London, UK.
- 289 University of Cordoba, Department of Cell Biology, Physiology and Immunology, Córdoba, Spain.
- 290 The Rockefeller University, Laboratory of Cellular and Molecular Neuroscience, New York, NY, USA.
- 291 Stockholm University, The Wenner-Gren Institute, Department of Molecular Biosciences, Stockholm, Sweden.
- 292 University of Verona, Department of Neurosciences, Biomedicine and Movement Sciences, Biological Chemistry Section, Verona, Italy.
- 293 Departmento de Biología Celular e Histología, Facultad de Biología, Universidad de Murcia, Instituto Murciano de Investigación Biosanitaria (IMIB)-Arrixaca, Murcia, Spain.
- 294 Universidad Nacional Autónoma de México, Faculty of Sciences, Department of Cell Biology, Fibrosis Lab, Mexico City, Mexico.
- 295 New York University School of Medicine, Skirball Institute and Department of Microbiology, New York, NY, USA.
- 296 Wenzhou Medical University, State Key Laboratory of Ophthalmology, Optometry and Visual Science, School of Optometry and Ophthalmology and the Eye Hospital, Wenzhou, China.
- 297 University of Louisville, Pediatric Research Institute, Department of Pediatrics, Louisville, KY, USA.
- 298 Rutgers University, The State University of New Jersey, Department of Cell Biology and Neuroscience, Piscataway, NJ, USA.
- 299 University of Barcelona, Department of Biochemistry and Molecular Biomedicine, and Network Center for Biomedical Research in Pathophysiology of Obesity and Nutrition (CIBEROBN), Barcelona, Spain.
- 300 University of Las Palmas de Gran Canaria, Department of Physical Education and Research Institute of Biomedical and Health Sciences (IUIBS), Las Palmas de Gran Canaria, Spain; Norwegian School of Sport Sciences, Department of Physical Performance, Oslo, Norway.
- 301 University of Alabama, Department of Biological Sciences, Tuscaloosa, AL, USA.
- 302 University of Georgia, Department of Kinesiology, Athens, GA, USA.
- 303 Fondazione Policlinico Universitario "Agostino Gemelli" IRCCS, Rome, Italy.
- 304 University of Zaragoza, Faculty of Veterinary-IIS Aragón, IA2-CITA, CIBERNED, Laboratory of Genetics and Biochemistry (LAGENBIO), Zaragoza, Spain.
- 305 University of São Paulo, Department of Immunology, Laboratory of Transplantation Immunobiology, São Paulo, SP, Brazil.
- 306 CNRS, Institut Curie, Paris, France.
- 307 University of Bordeaux, CNRS, IBGC, UMR 5095, Bordeaux, France.
- 308 University of London, The Royal Veterinary College, Department of Comparative Biomedical Sciences, UCL Consortium for Mitochondrial Research, London, UK.
- 309 Loyola University, Stritch School of Medicine, Department of Microbiology and Immunology, Chicago, IL, USA.
- 310 University of Ottawa, Department of Chemistry and Biomolecular Sciences and Department of Biochemistry, Microbiology and Immunology, Host-Microbe Interactions Laboratory, Ottawa, ON, Canada.
- 311 University of Rome "Tor Vergata", Department of Biology, Rome, Italy.
- 312 Università di Sassari, Dipartimento di Scienze Biomediche, Sassari, Italy.
- 313 Université Côte d'Azur, Université Nice Sophia Antipolis, CEA/DRF/BIAM, Faculté de Médecine, UMR E-4320 TIRO-MATOs, Nice, France.
- 314 Universidad San Sebastián, Centro de Biología Celular y Biomedicina (CEBICEM), Facultad de Medicina y Ciencia, Santiago, Chile.
- 315 University of Toronto, Sinai Health System, Lunenfeld-Tanenbaum Research Institute, Toronto, Ontario, Canada.
- 316 IRBLleida-University of Lleida,Department of Experimental Medicine, Lleida, Spain.
- 317 Xiamen University School of Medicine, Fujian Provincial Key Laboratory of Reproductive Health Research, Xiang'an, Xiamen, China.
- 318 University of Campania "L. Vanvitelli", Department of Precision Medicine, Naples, Italy; Institute of Genetic Research, Biogem scarl, Laboratory of Molecular and Precision Oncology, Ariano Irpino (AV), Italy.
- 319 Instituto de Investigación Biomédica de A Coruña (INIBIC), Complexo Hospitalario Universitario de A Coruña (CHUAC), Sergas, Universidade da Coruña (UDC), A Coruña, Spain.
- 320 University of Wisconsin, Department of Surgery, Madison, WI, USA.
- 321 University of Geneva, Department of Biochemistry, Faculty of Science, Switzerland.
- 322 Universidad Nacional Autónoma de México, Institute of Biotechnology, Plant Molecular Biology Department, Cuernavaca, Morelos, México.
- 323 University of Coimbra, Faculty of Medicine and CNC-Center for Neuroscience and Cell Biology, Coimbra, Portugal.
- 324 University of Arizona Cancer Center, Tucson, AZ, USA.
- 325 Université Côte d'Azur, Université Nice Sophia Antipolis, CEA/DRF/Institut Frédéric Joliot, Faculté de Médecine, UMR E-4320 TIRO-MATOs, Nice, France.
- 326 Trev and Joyce Deeley Research Centre, BC Cancer, Victoria, British Columbia, Canada.
- 327 University of Graz, Institute of Molecular Biosciences, NAWI Graz, Graz, Austria.
- 328 Federal University of Rio de Janeiro, Institute of Microbiology, Department of Immunology, Rio de Janeiro, Brazil.
- 329 Polytechnic University of Marche, Department of Life and Environmental Sciences, Ancona, Italy.
- 330 University of Modena and Reggio Emilia, Department of Biomedical, Metabolic and Neural Sciences, Modena, Italy.
- 331 Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR7258, Aix-Marseille Université U105, Institut Paoli-Calmettes, Parc Scientifique et Technologique de Luminy, Marseille, France.
- 332 Functional Genomics of Cardiomyopathies, Institute of Experimental Pharmacology and Toxicology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
- 333 University of Alabama at Birmingham, Department of Medicine, Pulmonary, Allergy, and Critical Care Medicine, Birmingham VA Medical Center, Birmingham, AL, USA.
- 334 Universidade de Lisboa, Faculty of Pharmacy, Research Institute for Medicines (iMed.ULisboa), Lisboa, Portugal.
- 335 Aix Marseille Univ, CNRS, LISM, Institut de Microbiologie de la Méditerranée, Marseille, France.
- 336 Universitat Autònoma de Barcelona, Institute of Neuroscience, Barcelona, Spain.
- 337 Institute for Advanced Chemistry of Catalonia (IQAC-CSIC), Department of Biological Chemistry; Networking Biomedical Research Centre on Hepatic and Digestive Diseases (CIBER-EHD), Barcelona, Spain.
- 338 University of New Mexico Health Sciences Center, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Albuquerque, NM, USA.
- 339 Universidad de Valparaíso, Centro Interdisciplinario de Neurociencia de Valparaíso, Laboratory of Molecular Sensors, Valparaíso, Chile.
- 340 Complutense University, Instituto de Investigaciones Sanitarias San Carlos (IdISSC), Department of Biochemistry and Molecular Biology, School of Biology, Madrid, Spain.
- 341 Université de Paris, Sorbonne Université, Centre de Recherche des Cordeliers, INSERM U1138, Team "Metabolism, Cancer & Immunity", Paris, France; Gustave Roussy Cancer Campus, Metabolomics and Cell Biology Platforms, Villejuif, France.
- 342 Division of Medical Genetics, Fondazione IRCCS-Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy.
- 343 Universidad de Concepción, Department of Biochemistry and Molecular Biology, Concepción, Chile.
- 344 Universidade NOVA de Lisboa, Faculdade de Ciências e Tecnologia, Departamento Ciências da Vida, UCIBIO, Caparica, Portugal.
- 345 Universidad de La Laguna, Departamento de Ciencias Médicas Básicas, Tenerife, Spain.
- 346 Universidad Nacional Autónoma de México (UNAM), Instituto de Fisiologia Celular, Ciudad Universitaria, Ciudad de México, México.
- 347 The Scripps Research Institute, Department of Molecular Medicine, La Jolla, CA, USA.
- 348 University of Coimbra, Portugal, CNC - Center for Neuroscience and Cell Biology & Faculty of Pharmacy, Coimbra, Portugal.
- 349 University of Pisa, Interdepartmental Research Centre on Biology and Pathology of Aging, Pisa, Italy.
- 350 University of Innsbruck, Institute for Biomedical Aging Research, Department of Molecular Biology, Innsbruck, Austria.
- 351 Biomedical Research Institute of Murcia, IMIB-Arrixaca, Telomerase, Cancer and Aging Group, Surgical Department, Murcia, Spain.
- 352 Research Center Borstel, Leibniz Lung Center, Priority Area Infections, Division Cellular Microbiology, Borstel, Germany.
- 353 Danish Cancer Society Research Center, Center for Autophagy, Recycling and Disease (CARD), Cell Stress and Survival Unit, Copenhagen, Denmark; IRCCS Bambino Gesù Children's Hospital, Department of Pediatric Hemato-Oncology and Cell and Gene therapy, Rome, Italy; University of Rome Tor Vergata, Department of Biology, Rome, Italy.
- 354 Medical University of Bialystok, Department of Pharmaceutical Biochemistry, Bialystok, Poland.
- 355 Ospedale San Raffaele and Vita-Salute San Raffaele University, Division of Genetics and Cell Biology, Milano, Italy.
- 356 Hospital Universitari de Tarragona Joan XXIII, Institut d'Investigació Sanitària Pere Virgili, Madrid, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
- 357 University of Minho, School of Medicine, Life and Health Sciences Research Institute (ICVS), Braga, Portugal.
- 358 Universidade Federal de São Paulo, Department of Morphology and Genetics, São Paulo, SP, Brazil.
- 359 University of Tuscia, Department for Innovation in Biological, Agro-food, and Forest systems (DIBAF), Viterbo, Italy.
- 360 Ege University, Faculty of Medicine, Department of Medical Biology, Izmir, Turkey.
- 361 School of Pharmacy, Jeonbuk National University, Jeollabuk-do, Republic of Korea.
- 362 Karolinska Institutet, Department of Physiology and Pharmacology, Stockholm, Sweden.
- 363 Kaohsiung Medical University, Department of Pathology, Kaohsiung City, Taiwan.
- 364 University of Calcutta, Department of Biotechnology and Dr. B.C. Guha Centre for Genetic Engineering and Biotechnology, Kolkata, India.
- 365 Saha Institute of Nuclear Physics, Biophysics & Structural Genomics Division, HBNI, Kolkata, India.
- 366 Ehime University, Department of Aquatic Life Sciences, Ainan, Ehime, Japan.
- 367 Justus-Liebig-University Giessen, Institute of Medical Microbiology, Giessen, Germany.
- 368 The University of Hong Kong, Li Ka Shing Faculty of Medicine, Department of Obstetrics and Gynecology, Hong Kong, China.
- 369 Queen's University, Department of Biomedical and Molecular Sciences, Kingston, Ontario, Canada.
- 370 The Chinese University of Hong Kong, School of Life Sciences, Shatin N.T., Hong Kong, China.
- 371 The Chinese University of Hong Kong, School of Biomedical Sciences, Hong Kong, China.
- 372 The Chinese University of Hong Kong, Department of Anaesthesia and Intensive Care, Hong Kong, China.
- 373 Tulane University School of Medicine, Department of Pharmacology, New Orleans, LA, USA.
- 374 National Cheng Kung University, College of Medicine, Department of Microbiology and Immunology, Tainan, Taiwan.
- 375 University of California, Berkeley, Department of Molecular Biology and Cell Biology, Berkeley, CA, USA; University of California, Berkeley, California Institute for Quantitative Biosciences, Berkeley, CA, USA.
- 376 National University of Singapore, Faculty of Sciences, Department of Pharmacy, Singapore.
- 377 Southeast University, School of Medicine, Department of Physiology, Nanjing, Jiangsu, China.
- 378 University of East Anglia, School of Biological Sciences, Norwich Research Park, UK.
- 379 Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), INSERM U964, CNRS UMR7104, University of Strasbourg, Illkirch, France.
- 380 NCR Biotech Science Cluster, Translational Health Science and Technology Institute, Complex Analysis Group, Faridabad, India.
- 381 Banaras Hindu University, Institute of Science, Department of Zoology, Lanka, Varanasi, Uttar Pradesh, India.
- 382 University of Washington, Department of Laboratory Medicine, Seattle, WA, USA.
- 383 Institute of Life Sciences, Bhubaneswar, Odisha, India.
- 384 UCL Institute of Ophthalmology, London, UK.
- 385 National Cheng Kung University, Department of Biochemistry and Molecular Biology, Tainan, Taiwan.
- 386 Academia Sinica, Institute of Biological Chemistry, Taipei, Taiwan.
- 387 University of Southern California, Center for Craniofacial Molecular Biology, Los Angeles, CA, USA.
- 388 University of Minnesota Medical School, Department of Laboratory Medicine and Pathology, Minneapolis, MN, USA.
- 389 Qingdao University, Institute of Brain Science and Disease, Qingdao, Shandong, China.
- 390 Army Medical University (Third Military Medical University), Daping Hospital, Center of Bone Metabolism and Repair, State Key Laboratory of Trauma, Burns and Combined Injury, Trauma Center, Research Institute of Surgery, Laboratory for the Prevention and Rehabilitation of Training Related Injuries, Chongqing, China.
- 391 Third Military Medical University, Southwest Hospital, Institute of Pathology and Southwest Cancer Centre, Chongqing, China.
- 392 Changhua Christian Hospital, Department of Otorhinolaryngology-Head and Neck Surgery, Changhua, Taiwan.
- 393 Wuhan Sports University, College of Health Science, Tianjiu Research and Development Center for Exercise Nutrition and Foods, Hubei Key Laboratory of Exercise Training and Monitoring, Wuhan, Hubei, China.
- 394 College of Life Sciences, Nankai University, Tianjin, China.
- 395 Wuhan University, School of Pharmaceutical Sciences, Zhongnan Hospital, Wuhan, China.
- 396 Duke University, Department of Medicine, Durham, NC, USA.
- 397 Chinese Academy of Sciences, Institute of Modern Physics, Lanzhou, China.
- 398 University of Sydney, Sydney Medical School, Renal Medicine, St Leonards, NSW, Australia.
- 399 Temple University, Lewis Katz School of Medicine, Cardiovascular Research Center and Department of Physiology, Philadelphia, PA, USA.
- 400 Chinese Academy of Medical Sciences and Peking Union Medical College, Institute of Dermatology, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Nanjing, Jiangsu, China.
- 401 Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, Shanghai, China.
- 402 Tsinghua University, School of Life Sciences, Beijing, China.
- 403 Peking University School of Basic Medical Science, Department of Immunology, Beijing, China.
- 404 Research Institute in Oncology and Hematology, CancerCare Manitoba, Winnipeg, Manitoba, Canada.
- 405 Mackay Memorial Hospital, Department of Radiation Oncology, Taipei, Taiwan.
- 406 Sun Yat-sen University, School of Life Science, Guangzhou, China.
- 407 Wuhan University, School and Hospital of Stomatology, Key Lab of Oral Biomedicine of Ministry of Education (KLOBME), Wuhan, Hubei, China.
- 408 2nd Affiliated Hospital of Zhejiang University School of Medicine, Department of Respiratory and Critical Care Medicine, Hangzhou, China.
- 409 University of Texas Southwestern Medical Center, Department of Molecular Biology, Dallas, Texas, USA.
- 410 Purdue University, Department of Botany and Plant Pathology, West Lafayette, IN, USA.
- 411 Wuhan University, Hubei Key laboratory of Cell Homeostasis, College of Life Sciences, Wuhan, China.
- 412 Capital Medical University, Beijing Ditan Hospital, Liver Disease Center, Beijing, China.
- 413 Northwestern University Feinberg School of Medicine, Department of Neurology & Lou & Jean Malnati Brain Tumor Institute, Chicago, IL, USA.
- 414 Albert Einstein College of Medicine, Department of Genetics, Bronx, NY, USA.
- 415 The University of Texas Health Science Center at Houston, Department of Integrative Biology and Pharmacology, Houston, TX, USA.
- 416 National Institutes of Health, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
- 417 East China Normal University, School of Life Sciences, Shanghai, China.
- 418 University of Florida, Food Science and Human Nutrition Department, Gainesville, FL, USA.
- 419 Zhejiang University, College of Pharmaceutical Sciences, Institute of Pharmacology and Toxicology, Hangzhou, Zhejiang, China.
- 420 National Cancer Center Korea, Goyang, Gyeonggi, Korea.
- 421 National University of Singapore, Yong Loo Lin School of Medicine, Department of Biochemistry, Singapore.
- 422 National University of Singapore, Yong Loo Lin School of Medicine, Strategic Research Programme in Precision Medicine, Singapore.
- 423 Lomonosov Moscow State University, A.N. Belozersky Institute of Physico-Chemical Biology, Moscow, Russia.
- 424 University of Pennsylvania, Department of Pathology and Laboratory Medicine, Philadelphia, PA, USA.
- 425 National Cheng Kung University, College of Medicine, Department of Pharmacology, Tainan, Taiwan.
- 426 Sun Yat-Sen University, School of Pharmaceutical Sciences, Guangzhou, China.
- 427 INSERM U1242, University of Rennes, Rennes, France; Centre de Lutte Contre le Cancer Eugène Marquis, Rennes, France.
- 428 University of North Carolina at Charlotte, Department of Biological Sciences, Charlotte, NC, USA.
- 429 The University of Hong Kong, Queen Mary Hospital, Department of Paediatrics and Adolescent Medicine, Pokfulam, Hong Kong, China.
- 430 University of Milano-Bicocca, Department of Biotechnology and Biosciences, Milan, Italy.
- 431 Istituto di Fisiologia Clinica, Consiglio Nazionale delle Ricerche, Siena, Italy; Core Research Laboratory, Istituto per lo Studio, la Prevenzione e la Rete Oncologica, Siena, Italy.
- 432 The Norwegian Radium Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Montebello, Oslo, Norway; University of Oslo, Faculty of Mathematics and Natural Sciences, The Department of Biosciences, Oslo, Norway.
- 433 The Norwegian Radium Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Montebello, Oslo, Norway; University of Oslo, Faculty of Medicine, Institute of Clinical Medicine, Centre for Cancer Cell Reprogramming, Oslo, Norway.
- 434 IEO, European Institute of Oncology IRCCS, IFOM-IEO Campus, Department of Experimental Oncology, Milan, Italy.
- 435 University of Chile, Faculty of Chemical and Pharmaceutical Sciences, Advanced Center for Chronic Diseases (ACCDiS), Santiago, Chile.
- 436 Taipei Veterans Generals Hospital, Department of Medical Research, Taipei, Taiwan.
- 437 National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Rocky Mountain Laboratories (RML), Laboratory of Virology, Innate Immunity and Pathogenesis Section, Hamilton, MT, USA.
- 438 Institute of Translational Pharmacology, National Research Council, Rome, Italy; European Center for Brain Research, Laboratory of Resolution of Neuroinflammation, IRCCS Santa Lucia Foundation, Rome, Italy.
- 439 Kyungpook National University, Department of Life Science, Deagu, Korea.
- 440 Wonkwang University School of Medicine, Department of Microbiology, Iksan, Jeonbuk, Korea.
- 441 Weill Cornell Medicine, Department of Medicine, New York, NY, USA.
- 442 Weill Cornell Medicine, Division of Nephrology and Hypertension, Joan and Sanford I. Weill Department of Medicine, New York, NY, USA.
- 443 CSIR-Indian Institute of Chemical Biology, Kolkata, India.
- 444 BC Cancer Research Centre, Department of Experimental Therapeutics, Vancouver, BC, Canada.
- 445 University of Pittsburgh School of Medicine, Department of Pathology, Pittsburgh, PA, USA.
- 446 National University of Singapore, Yong Loo Lin School of Medicine, Department of Physiology; National University of Singapore, LSI Neurobiology Programme; National University of Singapore, Institute for Health Innovation and Technology; National University Hospital Singapore, Centre for Healthy Longevity; Institute of Molecular and Cell Biology, Agency for Science, Technology and Research (A*STAR), Singapore.
- 447 Konkuk University School of Medicine, Department of Ophthalmology, Seoul, Korea.
- 448 Max-Planck-Institut für Molekulare Pflanzenphysiologie, Potsdam, Germany.
- 449 University of Ulsan College of Medicine, Asan Medical Center, Department of Psychiatry, Seoul, Korea.
- 450 The Catholic University of Korea, Seoul St, Mary's Hospital, Department of Ophthalmology and Visual Science, Seoul, Kor.
- 451 Institute of Cancer Research, Cancer Research UK Cancer Imaging Centre, Division of Radiotherapy and Imaging, London, UK.
- 452 Nencki Institute of Experimental Biology of Polish Academy of Sciences, Neurobiology Center, Laboratory of Molecular Neurobiology, Warsaw, Poland.
- 453 University of Chile, Institute of Nutrition and Food Technology (INTA), Advanced Center for Chronic Diseases (ACCDiS), Santiago, Chile.
- 454 University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Pediatrics, Cologne, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Center for Molecular Medicine (CMMC), Cologne, Germany.
- 455 University of Rome "La Sapienza", Rome, Italy.
- 456 University of Liverpool, Institute of Translational Research, Cellular and Molecular Physiology, Liverpool, UK.
- 457 Molecular Physiology and Cell Signaling, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK.
- 458 Università di Milano, Department of Biomedical and Clinical Sciences L. Sacco, Milan, Italy.
- 459 Istituto Superiore di Sanità, Department of Infectious Diseases, Rome, Italy.
- 460 Institut Necker-Enfants Malades (INEM), INSERM U1151/CNRS UMR 8253, Université de Paris, Paris, France.
- 461 The Hebrew University of Jerusalem, The Institute for Medical Research Israel-Canada, Department of Biochemistry and Molecular Biology, Ein Karem, Jerusalem, Israel.
- 462 University Roma Tre, Department of Science, LIME, Rome, Italy.
- 463 National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Pediatric Translational Research Branch, Bethesda, MD, USA.
- 464 Institut d'Investigacions Biomèdiques de Barcelona (CSIC, IDIBAPS), and Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Barcelona, Spain.
- 465 University of Belgrade, Institute for the Application of Nuclear Energy (INEP), Belgrade, Serbia.
- 466 Centre for Research in Agricultural Genomics (CRAG), CSIC-IRTA-UAB-UB, Campus UAB, Bellaterra, Barcelona, Spain.
- 467 University of Sheffield, Department of Biomedical Science, Firth Court, Western Bank, Sheffield, UK.
- 468 Instituto de Histología y Embriología (IHEM)-Universidad Nacional de Cuyo-CONICET, Facultad de Ciencias Médicas, Mendoza, Argentina.
- 469 Yale University School of Medicine, Department of Neuroscience and Department of Cell Biology, New Haven, CT, USA.
- 470 Clermont-Auvergne University, INRAE, Human Nutrition Unit, F-63000, Clermont-Ferrand, France.
- 471 University of Pavia, Department of Biology and Biotechnology "L. Spallanzani", Pavia, Italy.
- 472 Federal University of São Carlos, Department of Gerontology, São Carlos, SP, Brazil.
- 473 Department of Pathology and Experimental Therapeutics and Institute of Biomedicine of the University of Barcelona (IBUB), Bellvitge University Hospital-IDIBELL, Hospitalet de Llobregat; University of Brescia, Department of Molecular and Translational Medicine, Brescia BS, Italy.
- 474 National Institutes of Health, NIAMS, Lymphocyte Nuclear Biology, Bethesda, MD, USA.
- 475 Rutgers University, Department of Biological Sciences, Newark, NJ, USA.
- 476 National Institutes of Health, National Institute on Aging, Laboratory of Neurogenetics, Bethesda, MD, USA.
- 477 University of Manitoba, Department of Medical Microbiology & Infectious Diseases, Winnipeg, Manitoba, Canada.
- 478 Johns Hopkins University, Department of Molecular Microbiology and Immunology, Baltimore, MD, USA.
- 479 University Magna Graecia of Catanzaro, Department of Health Sciences, Catanzaro, Italy.
- 480 Umeå University, Umeå Centre for Microbial Research (UCMR), Department of Chemistry, Umeå, Sweden.
- 481 OncoRay National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany; Helmholtz-Zentrum Dresden - Rossendorf, Institute of Radiooncology - OncoRay, Dresden, Germany; German Cancer Consortium (DKTK), Partner site Dresden, Germany; German Cancer Research Center (DKFZ), Heidelberg, Germany.
- 482 OncoRay National Center for Radiation Research in Oncology, Faculty of Medicine, Technische Universität Dresden, Dresden, Germany.
- 483 University of Genoa, DIMES Department of Experimental Medicine, Human Anatomy, Genoa, Italy.
- 484 University of Michigan Medical School, Department of Neurology, Ann Arbor, MI, USA.
- 485 University of Zürich, Schlieren Campus, Center for Molecular Cardiology, Schlieren, Switzerland.
- 486 University of Oviedo, Department of Morphology and Cell Biology, Oviedo, Spain.
- 487 University of Melbourne, Department of Pharmacology and Therapeutics, Melbourne, Victoria, Australia.
- 488 CSIC-Universidad de Sevilla, Instituto de Bioquímica Vegetal y Fotosíntesis, Sevilla, Spain.
- 489 Universidad de Chile, Facultad de Odontología, Advanced Center for Chronic Diseases, Santiago, Chile.
- 490 Università degli Studi di Milano, Dipartimento di Scienze Farmacologiche e Biomolecolari, Centro di Eccellenza per lo studio delle Malattie Neurodegenerative, Milano, Italy.
- 491 Department of Anatomy, Cell and Developmental Biology, Eötvös Loránd University, Budapest, Hungary.
- 492 Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Instituto de Investigación Sanitaria La Paz (IdiPaz), Instituto de Investigaciones Biomédicas Alberto Sols UAM-CSIC, Madrid, Spain; Universidad Autónoma de Madrid, School of Medicine, Department of Biochemistry, Madrid, Spain.
- 493 Chinese Academy of Medical Sciences & Peking Union Medical College, Institute of Materia Medica, State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Molecular Immunology and Cancer Pharmacology Group, Beijing, China.
- 494 Sun Yat-sen University, School of Life Sciences, MOE Key Laboratory of Gene Function and Regulation, Guangzhou, Guangdong, China.
- 495 Medical Research Institute, Wuhan University, Wuhan, China.
- 496 The Chinese University of Hong Kong, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, Shatin, New Territories, Hong Kong, China.
- 497 Université Paris-Saclay, CEA, 1035 CNRS, Institute for Integrative Biology of the Cell (I2BC), INSERM U1280, Gif-sur-Yvette, France.
- 498 University of Mar del Plata, Department of Biology and Chemestry, Mar del Plata, BA, Argentina.
- 499 McGill University Health Centre, Montréal, QC, Canada.
- 500 Emory University School of Medicine, Department of Medicine, Atlanta, GA, USA.
- 501 Medical University of Lublin, Department of Pathophysiology, Lublin, Poland.
- 502 University of Bologna, Department of Medical and Surgical Sciences; Rizzoli Orthopaedics Institute, Laboratory of Immunorheumatology and Tissue Regeneration, Bologna, Italy.
- 503 University Campus Bio-Medico, Department of Medicine, Rome, Italy.
- 504 Istituto Dermopatico dell'Immacolata, IDI-IRCCS, Rome, Italy.
- 505 University of Florida, Department of Physical Therapy, Gainesville, FL, USA.
- 506 University "G. D'Annunzio", Department of Medical Sciences DSMOB, Chieti, Italy; Regina Elena National Cancer Institute, Department of Research, Rome, Italy.
- 507 Federal University of Sergipe, Department of Pharmacy, São José, SE, Brazil.
- 508 University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Pediatrics, Cologne, Germany.
- 509 University of Nevada, Reno School of Medicine, Department of Pharmacology, Reno, NV, USA.
- 510 Gregor Mendel Institute, Vienna Biocenter, Vienna, Austria.
- 511 University of Naples Federico II, Department of Pharmacy, Naples, Italy.
- 512 Xi'an Jiaotong University, Department of Public Health, Xi'an, Shaanxi, China.
- 513 The First Hospital of Jilin University, Laboratory of Cancer Precision Medicine, Changchun, Jilin, China.
- 514 Model Animal Research Center of Nanjing University, Nanjing, Jiangsu, China.
- 515 University of Salerno, Department of Medicine, Surgery and Dentistry "Scuola Medica Salernitana", Baronissi, SA, Italy.
- 516 University of Padova, Department of Biology, Padova, Italy.
- 517 Research Center Borstel, Cellular Microbiology, Borstel, Germany.
- 518 University of Clermont Auvergne, M2iSH (Microbes, Intestine, Inflammation and Susceptibility of the Host), UMR 1071 Inserm, INRA USC 2018, CRNH, Clermont-Ferrand, France.
- 519 Christian-Albrechts-University Kiel, Biochemical Institute, Kiel, Germany.
- 520 Karolinska Institutet, Biomedicum, Department of Cell and Molecular Biology, Stockholm, Sweden.
- 521 University of South Florida, Morsani College of Medicine, Department of Molecular Medicine and USF Health Byrd Alzheimer's Research Institute, Tampa, FL, USA; Institute for Biological Instrumentation of Russian Academy of Sciences, Laboratory of New Methods in Biology, Pushchino, Moscow region, Russia.
- 522 University of South Florida, Morsani College of Medicine, Department of Molecular Medicine, Tampa, FL, USA.
- 523 Texas Tech University Health Sciences Center, Department of Pharmaceutical Sciences, Amarillo, TX, USA.
- 524 Cleveland Clinic, Department of Gastroenterology, Hepatology and Nutrition, Cleveland, OH, USA.
- 525 MD Anderson Cancer Center, Department of Gynecologic Oncology and Reproductive Medicine, Houston, TX, USA.
- 526 Tulane University Health Sciences Center, Department of Pathology and Laboratory Medicine, New Orleans, LA, USA.
- 527 Max-Delbrück-Center for Molecular Medicine, Department of Crystallography, Berlin, Germany.
- 528 Institute of Life Sciences, School of Medicine, Swansea University, Swansea, Wales, UK.
- 529 University of Massachusetts Medical School, Program in Molecular Medicine, Worcester, MA, USA.
- 530 Stellenbosch University, Department of Physiological Sciences, Stellenbosch, Western Cape, South Africa.
- 531 University of Dundee School of Medicine, Division of Cellular Medicine, Dundee, Scotland, UK.
- 532 University of Calabria, Department of Farmacy Health and Nutritional Sciences, Rende, Italy.
- 533 Ghent University, Translational Nuclear Receptor Research, VIB Center for Medical Biotechnology, Department of Biomolecular Medicine, Ghent, Belgium.
- 534 "Sapienza" University of Rome, Department of Radiotherapy, Policlinico Umberto I, Rome, Italy.
- 535 University of Verona, Azienda Ospedaliera Universitaria Integrata-Verona, Department of Medicine, Verona; Italy.
- 536 Oswaldo Cruz Foundation, Oswaldo Cruz Institute, Leprosy Laboratory, Rio de Janeiro, Brazil.
- 537 University of Antwerp, Laboratory of Physiopharmacology, Antwerp, Belgium.
- 538 Department of Oncology-Pathology, Cancer Center Karolinska, Karolinska Institute, Stockholm, Sweden.
- 539 Sapienza University of Rome, Ospedale Sant'Andrea, Rome, Italy.
- 540 Università degli Studi di Milano, Department of Medical Biotechnology and Translational Medicine, Milano, Italy.
- 541 University of Urbino Carlo Bo, Department of Biomolecular Sciences, Unit of Pharmacology and Public Health, Urbino, Italy.
- 542 University of Fribourg, Department of Biology, Fribourg, Switzerland.
- 543 Danish Cancer Society Research Center, Cell Stress and Survival Unit, Center for Autophagy, Recycling and Disease (CARD), Copenhagen, Denmark.
- 544 University of California, San Francisco, Department of Pathology, San Francisco, CA, USA.
- 545 Washington University School of Medicine, Departments of Pediatrics and Cell Biology & Physiology, St. Louis, MO, USA.
- 546 KU Leuven, Department of Development and Regenaration, Laboratory of Pediatrics, PKD Research Group, Leuven, Belgium.
- 547 Boston University, Department of Biology, Boston, MA, USA.
- 548 IFOM, The FIRC Institute of Molecular Oncology, Milan, Italy.
- 549 University of Colorado Anschutz Medical Campus, Department of Biochemistry and Molecular Genetics, Aurora, CO, USA.
- 550 University of Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
- 551 Instituto de Fisiologia Celular, UNAM, Department of Biochemistry and Structural Biology, Mexico City, Mexico.
- 552 Medical University of South Carolina, Charleston, SC 29425, USA.
- 553 University of Melbourne, School of Biomedical Sciences, Melbourne, Victoria, Australia.
- 554 University of Buenos Aires, Faculty of Pharmacy and Biochemistry, Institute of Immunology, Genetics and Metabolism (INIGEM), CONICET. Buenos Aires, Argentina.
- 555 L.N.C.I.B. Laboratorio Nazionale Consorzio Interuniversitario Biotecnologie, AREA Science Park, Trieste, Italy.
- 556 University of Zagreb School of Medicine, Department of Physiology and Croatian Institute for Brain Research, Zagreb, Croatia.
- 557 Hospital for Sick Children, Program in Cell Biology Department, Toronto, Canada; University of Toronto, Department of Biochemistry, Toronto, Canada.
- 558 Chinese Academy of Medical Sciences & Peking Union Medical College, Institute of Medicinal Biotechnology, Beijing, China.
- 559 Virginia Commonwealth University, Department of Biochemistry and Molecular Biology, Richmond, VA, USA.
- 560 Centre for Cancer Biology, University of South Australia, Adelaide, Australia.
- 561 Division of Developmental Biology, National Institute of Child Health & Human Development, National Institutes of Health, Bethesda, MD, USA.
- 562 University of North Carolina at Chapel Hill, Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA.
- 563 University of New Mexico Health Sciences Center, Autophagy, Inflamamtion and Metabolism (AIM) Center, Albuquerque, NM, USA.
- 564 Institut National de la Recherche Scientifique, Centre Armand-Frappier Santé Biotechnologie, Laval, QC, Canada.
- 565 Bellvitge Biomedical Research Institute-IDIBELL, Oncobell Program. L'Hospitalet de Llobregat, Barcelona, Spain.
- 566 University of California, San Diego; Section of Cell and Developmental Biology, La Jolla, CA, USA.
- 567 University of Zurich, Institute of Physiology, Zurich, Switzerland.
- 568 Walter and Eliza Hall Institute of Medical Research, Ubiquitin Signalling Division, Melbourne, Australia; University of Melbourne, Department of Medical Biology, Melbourne, Australia.
- 569 University of Naples "Federico II", Department of Chemical, Materials and Industrial Production Engineering, Naples, Italy.
- 570 University of Perugia, Department of Chemistry, Biology and Biotechnology, Perugia, Italy.
- 571 Sapienza University of Rome, Department of Biochemical Sciences A. Rossi Fanelli, Rome, Italy.
- 572 Philipps University of Marburg, Department of Visceral, Thoracic and Vascular Surgery, Marburg, Germany.
- 573 IRCCS Foundation Ca' Granda Ospedale Maggiore Policlinico, Dino Ferrari Center, Neuroscience Section, University of Milan, Department of Pathophysiology and Transplantation, Milan, Italy.
- 574 University of Teramo, Faculty of Veterinary Medicine, Teramo, Italy.
- 575 Western University, Department of Physiology and Pharmacology, London, ON, Canada.
- 576 Harvard Medical School, Boston Children's Hospital, F.M. Kirby Neurobiology Center, Translational Neuroscience Center, Department of Neurology, Boston, MA, USA.
- 577 National Institute for Infectious Diseases "L. Spallanzani" IRCCS, Rome, Italy.
- 578 National and Kapodistrian University of Athens, Department of Biology, Panepistimioupolis, Athens, Greece.
- 579 University of Cincinnati College of Medicine, Department of Cancer Biology, Cincinnati, OH, USA.
- 580 University of Chile, Faculty of Chemical and Pharmaceutical Sciences, Department of Chemical Pharmacology and Toxicology, Santiago de Chile, Chile.
- 581 University of Alcalá, Deparment of Systems Biology, Biochemistry and Molecular Biology, Alcalá de Henares, Madrid, Spain.
- 582 Arizona State University, Phoenix, AZ, USA.
- 583 Seoul National University, College of Pharmacy, Seoul, Korea.
- 584 University of Montreal, Faculty of Medicine, Microbiology, Infectious diseases and Immunology Department, CRCHUM, Montréal, QC, Canada.
- 585 Goethe University, Institute of Biochemistry II, Faculty of Medicine, Frankfurt am Main, Germany.
- 586 Zhejiang University, School of Medicine, Department of Cell Biology, Hangzhou, Zhejiang Province, China.
- 587 University of Kansas Medical Center, Department of Pharmacology, Toxicology and Therapeutics, Kansas City, KS, USA.
- 588 University of Rome La Sapienza, Department of Biology and Biotechnology C. Darwin, Rome, Italy.
- 589 University of Belgrade, Institute for Biological Research "Sinisa Stankovic" - National Institute of Republic of Serbia, Department of Neurobiology, Belgrade, Serbia.
- 590 University of Belgrade, Institute of Molecular Genetics and Genetic Engineering, Belgrade, Serbia.
- 591 Johns Hopkins University School of Medicine, Department of Pharmacology and Molecular Sciences and Department of Medicine, Baltimore, MD, USA.
- 592 Imperial College London, Department of Life Sciences and MRC Centre for Molecular Bacteriology and Infection, London, UK.
- 593 Friedrich-Alexander University (FAU) Erlangen-Nürnberg and Universitätsklinikum Erlangen, Department of Internal Medicine 3 - Rheumatology and Immunology, Erlangen, Germany.
- 594 Washington University School of Medicine and John Cochran VA Medical Center, Department of Medicine, St. Louis, MO, USA.
- 595 University of Manitoba, Institute of Cardiovascular Sciences, Department of Physiology and Pathophysiology, Winnipeg, Manitoba, Canada.
- 596 Centre de Recherche des Cordeliers, INSERM UMRS 1138, Sorbonne Université, Université de Paris, Equipe 11 labellisée par la Ligue contre le Cancer, 75006 Paris, France; Metabolomics and Cell Biology Platforms, Institut Gustave Roussy, 94805 Villejuif, France.
- 597 University of Calgary, Department of Comparative Biology and Experimental Medicine, Calgary, AB, Canada.
- 598 University of Colima, University Center for Biomedical Research, Colima, Mexico.
- 599 University of Canterbury, Biomolecular Interaction Centre, School of Biological Sciences, Christchurch, New Zealand.
- 600 Hacettepe University, Faculty of Medicine, Department of Medical Biology, Ankara, Turkey.
- 601 University of Kentucky, College of Medicine, Markey Cancer Center, Lexington, KY, USA.
- 602 University of Texas Southwestern Medical Center, Department of Internal Medicine, Center for Autophagy Research, Dallas, TX, USA.
- 603 Jinshan Branch of Shanghai Sixth People's Hospital, Department of Laboratory Medicine, Shanghai, China.
- 604 Washington University in St Louis School of Medicine, Department of Internal Medicine, St. Louis, MO, USA.
- 605 Goethe University, Institute of Biophysical Chemistry, Frankfurt, Germany.
- 606 Nanjing University, Medical School, Division of Immunology, Nanjing, Jiangsu, China.
- 607 Emory University School of Medicine, Department of Psychiatry and Behavioral Sciences, Atlanta, GA, USA.
- 608 Umeå University, Department of Chemistry, Umeå, Sweden.
- 609 University of Arkansas, Center of Excellence for Poultry Science, Fayetteville, AR, USA.
- 610 Oncogenetic Laboratory, Meir Medical Center, Kfar Saba, and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
- 611 Tongren Hospital, Shanghai Jiaotong University School of Medicine, Department of Neurology, Shanghai, China.
- 612 University of British Columbia, Department of Urologic Sciences, Vancouver, BC, Canada.
- 613 National Institute of Biological Sciences, Beijing, China.
- 614 Central South University, The Second Xiangya Hospital, Department of Nephrology, Changsha , Hunan, China.
- 615 Chinese Academy of Medical Sciences and Peking Union Medical College, Institute of Blood Transfusion, Chengdu, China.
- 616 Cardiff University, Division of Cancer and Genetics, Heath Park, Cardiff, UK.
- 617 Centro Andaluz de Biología Molecular y Medicina Regenerativa CABIMER, Consejo Superior de Investigaciones Científicas, Universidad de Sevilla, Universidad Pablo de Olavide, Seville, Spain.
- 618 Shanghai Proton and Heavy Ion Center, Department of Research & Development, Pudong, Shanghai, China.
- 619 University Medical Center Hamburg-Eppendorf, Department of Oncology, Hematology and Bone Marrow Transplantation with Section Pneumology, Hamburg, Germany.
- 620 A.V. Zhirmunsky National Scientific Center of Marine Biology, Laboratory of Pharmacology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russian Federation.
- 621 Far Eastern Federal University, School of Natural Sciences, Vladivostok, Russian Federation.
- 622 Department of Neurology, Boston Children's Hospital, Boston, MA, USA.
- 623 Medical University of Vienna, Department of Dermatology, Division for Biology and Pathobiology of the Skin, Vienna, Austria.
- 624 University of Colorado Anschutz Medical Campus, Division of Renal diseases, Aurora, CO, USA.
- 625 University of Manitoba, Department of Physiology and Pathophysiology, Regenerative Medicine Program, Winnipeg, Manitoba, Canada.
- 626 University of Cologne, Centre for Biochemistry, Institute of Biochemistry I, Medical Faculty, Cologne, Germany.
- 627 United Arab Emirates University, College of Medicine & Health Sciences, Department of Anatomy, Al Ain, UAE.
- 628 University of Graz, NAWI Graz, Institute of Molecular Biosciences, Graz, Austria; BioTechMed Graz, Graz, Austria; University of Graz, Field of Excellence BioHealth, Graz, Austria.
- 629 Long Beach VA and University of California, Irvine, Irvine, CA, USA.
- 630 Ain Shams University, Faculty of Medicine, Department of Medical Biochemistry and Molecular Biology, Cairo, Egypt.
- 631 Hospital Universitari de Tarragona Joan XXIII, Institut d'Investigació Santitària Pere Virgili.
- 632 University of Illinois at Chicago, Department of Pathology, College of Medicine, Chicago, IL, USA.
- 633 Florida International University, Department of Immunology and Nano-Medicine, Miami, FL, USA.
- 634 Children's Cancer Hospital Egypt 57357, Tumor Biology Research Program, Cairo, Egypt.
- 635 University of Camerino, Department of Biosciences and Biotechnology, Camerino, Italy.
- 636 Damietta University, Faculty of Science, Biochemistry Department, Damietta, Egypt.
- 637 Technische Universität Dresden, Medical Clinic I, University Hospital Carl Gustav Carus, Dresden, Germany; Technische Universität Dresden, Faculty of Medicine, Institute for Clinical Chemistry and Laboratory Medicine, Dresden, Germany; Institute of Molecular Genetics of the ASCR, Department of Cancer Cell Biology, Prague, Czech Republic.
- 638 Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (INBIRS), Universidad de Buenos Aires-CONICET, Argentina.
- 639 University of Sheffield, The Bateson Centre, Department of Infection, Immunity and Cardiovascular Disease,Sheffield, South Yorkshire, UK.
- 640 Philipps University of Marburg, Department of Cytobiology and Cytopathology, Marburg, Germany.
- 641 Sanford Burnham Prebys Medical Discovery Research Institute, La Jolla, CA, USA.
- 642 Bogazici University, Department of Molecular Biology and Genetics, Bebek, Istanbul, Turkey.
- 643 Pfizer, Oncology Research & Development, Pearl River, NY, USA.
- 644 Oslo University Hospital, Institute for Cancer Research, Department of Tumor Biology, Montebello, Oslo, Norway.
- 645 University of Bergen, Department of Biomedicine/ Centre for Cancer Biomarkers (CCBIO), Bergen, Norway.
- 646 Instituto de Investigaciones Biomédicas Alberto Sols, C.S.I.C./U.A.M., Madrid, Spain.
- 647 Universität zu Köln, CECAD Forschungszentrum, Institut für Genetik, Köln, Germany.
- 648 University of Turku, Institute of Biomedicine, Turku, Finland.
- 649 IRIM, University of Montpellier, CNRS, Montpellier, France.
- 650 Medical University of Lodz, Department of Laboratory Diagnostics, Lodz, Poland.
- 651 Sapienza University of Rome, DAHFMO-Section of Anatomy, Rome, Italy.
- 652 Istituto Superiore di Sanità, Department of Oncology and Molecular Medicine, Rome, Italy.
- 653 Karolinska Institutet, Institute of Environmental Medicine, Stockholm, Sweden.
- 654 Universidad Nacional de Cuyo, Facultad de Ciencias Médicas, Laboratorio de Biología Celular y Molecular, IHEM-CONICET, Mendoza, Argentina.
- 655 Max-Planck-Institute of Biophysical Chemistry, Biochemistry of Signal Dynamics, Göttingen, Germany.
- 656 La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria, Australia; Olivia Newton-John Cancer Research Institute, Heidelberg, Victoria, Australia; School of Cancer Medicine, La Trobe University, Melbourne, Victoria, Australia.
- 657 Universidad Miguel Hernández (UMH), Instituto de Investigación, Desarrollo e Innovación en Biotecnología Sanitaria de Elche (IDiBE), Elche, Spain.
- 658 Dresden University Medical Center, Department of Neurology, Dresden, Germany.
- 659 University of South Carolina School of Medicine, Department of Cell Biology and Anatomy, Columbia, SC, USA.
- 660 University of Pittsburgh, Department of Surgery, Pittsburgh, PA, USA.
- 661 University of Cincinnati, Department of Cancer Biology, Cincinnati, OH, USA.
- 662 University of Oslo and Akershus University Hospital, Department of Clinical Molecular Biology, Lørenskog, Norway; The Norwegian Centre on Healthy Ageing (NO-Age), Oslo, Norway.
- 663 Shanghai Institute of Organic Chemistry, Chinese Academy of Science, Interdisciplinary Research Center on Biology and Chemistry, Shanghai, China; University of Chinese Academy of Sciences, Beijing, China.
- 664 The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China.
- 665 King's College London, Department of Basic and Clinical Neuroscience, London, United Kingdom; Institut du Cerveau et de la Moelle épinière (ICM), Paris, France.
- 666 Centre International de Recherche en Infectiologie (CIRI), University Lyon, Inserm, U1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, ENS Lyon, Lyon, France.
- 667 Rudolf Virchow Center, University of Würzburg, Würzburg, Germany.
- 668 South Australian Health and Medical Research Institute, Hopwood Centre for Neurobiology, Lifelong Health Theme, Adelaide, South Australia, Australia.
- 669 Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA.
- 670 Guangzhou Medical University, School of Basic Medical Sciences, State Key Laboratory of Respiratory Disease, Guangzhou, China.
- 671 The First Hospital of Jilin University, Department of Neurology, Changchun, China.
- 672 Medical School of Zhejiang University, Sir Run Run Shaw Hospital, Key lab of Biotherapy in Zhejiang, Laboratory of Cancer Biology, Hangzhou, China.
- 673 The University of Hong Kong, School of Chinese Medicine, Li Ka Shing Faculty of Medicine, Hong Kong, China.
- 674 Baylor College of Medicine, Department of Biochemistry and Molecular Biology, Houston TX, USA.
- 675 Chinese Academy of Sciences, Institute of Biophysics, Beijing, China.
- 676 University of São Paulo (USP), Ribeirão Preto Medical School, Department of Genetics, Ribeirão Preto, SP, Brazil.
- 677 Washington University in St. Louis, Department of Ophthalmology and Visual Sciences, St. Louis, MO, USA.
- 678 University of Texas Southwestern Medical Center, Center for Autophagy Research, Department of Internal Medicine and Howard Hughes Medical Institute, Dallas, TX, USA.
- 679 Institute of Biomedical Research of Barcelona (IIBB)-CSIC, Barcelona, Spain; Liver Unit, Hospital Clinic i Provincial de Barcelona, IDIBAPS and CIBEREHD, Barcelona, Spain; Research Center for ALPD, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
- 680 Columbia University, New York, NY, USA.
- 681 Università del Piemonte Orientale, Department of Health Sciences, Laboratory of Molecular Pathology, Novara, Italy.
- 682 University of Sao Paulo, Institute of Biosciences, Sao Paulo, SP, Brazil.
- 683 University of California, San Diego, Department of Cellular and Molecular Medicine, La Jolla, CA, USA.
- 684 Instituto de Investigaciones Marinas CSIC, Department of Biotechnology and Aquaculture, Immunology and Genomics, Vigo, Spain.
- 685 University of Padua, Department of Biomedical Sciences, Padua, Italy; Italian National Research Council (CNR), Neuroscience Institute, Padua, Italy.
- 686 University of Piemonte Orientale, Department of Translational Medicine, Novara, Italy.
- 687 Universidade Federal do Rio Grande do Sul (UFRGS), Morphological Sciences Department, Porto Alegre, RS, Brazil; Clinicas Hospital of Porto Alegre, Porto Alegre, RS, Brazil.
- 688 Danish Cancer Society Research Center, Redox Signaling and Oxidative Stress Group, Copenhagen, Denmark.
- 689 Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.
- 690 Università degli Studi Sapienza di Roma, SAIMLAL Department, Roma, Italy.
- 691 Center for Systems and Therapeutics, Gladstone Institutes; Departments of Neurology and Physiology, University of California San Francisco, San Francisco, CA, USA.
- 692 NYU School of Medicine, Department of Medicine, New York, NY, USA.
- 693 Virginia Commonwealth University, School of Medicine, Department of Human and Molecular Genetics, Richmond, VA, USA; Virginia Commonwealth University, VCU Institute of Molecular Medicine, Richmond, VA, USA; Columbia University, College of Physicians and Surgeons, Departments of Pathology, Neurosurgery and Urology, New York, NY, USA.
- 694 State University of New York, University at Buffalo, Jacobs School of Medicine and Biomedical Sciences, Departments of Ophthalmology/Biochemistry and Neuroscience Program, Buffalo, NY, USA.
- 695 Norwegian University of Science and Technology, Department of Clinical and Molecular Medicine, Centre for Molecular Inflammation Research, Trondheim, Norway.
- 696 Central University of Tamil Nadu, Thiruvarur, Tamil Nadu, India.
- 697 Babraham Institute, Signalling ISP, Cambridge, UK.
- 698 University of Genova, Department of Internal Medicine and IRCCS Policlinico San Martino, Genova, Italy.
- 699 Montreal Neurological Institute, Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada.
- 700 University of Pavia, Department of Molecular Medicine, Biochemistry Unit, Pavia, Italy.
- 701 University of Pisa, Department of Translational Medicine and New Technologies in Medicine and Surgery, Pisa, Italy; I.R.C.C.S. Neuromed Pozzilli, Pozzilli, Italy.
- 702 Istituto Superiore di Sanità, Department of Environment and Health, Section of Mechanisms, Biomarkers and Models, Rome, Italy.
- 703 University of Texas Medical Branch, Mitchell Center for Neurodegenerative Diseases, Department of Neurology, Galveston, TX, USA.
- 704 University of Nebraska-Lincoln, Redox Biology Center, Lincoln, NE, USA.
- 705 Laboratory of Sex-gender Medicine, National Institute of Biostructures and Biosystems, Sassari, Italy.
- 706 Icahn School of Medicine at Mount Sinai, Department of Medicine, Division of Liver Diseases, New York, NY, USA.
- 707 Institute of Science and Technology Austria (IST), Klosterneuburg, Austria.
- 708 Clínica Universidad de Navarra, Metabolic Research Laboratory, CIBEROBN, IdiSNA, Pamplona, Spain.
- 709 University of Extremadura, Centro de Investigación Biomedica en Red de Enfermedades Neurodegenerativas (CIBERNED), Instituto de Investigación Biosanitaria de Extremadura (INUBE), Department of Biochemistry, Molecular Biology and Genetics, Faculty of Nursing and Occupational Therapy, Cáceres, Spain.
- 710 Kyushu University, Medical Institute of Bioregulation, Fukuoka, Japan.
- 711 Tokyo Institute of Technology, Institute of Innovative Research, Cell Biology Center, Yokohama, Japan.
- 712 National Institute of Neuroscience, National Center of Neurology and Psychiatry, Department of Degenerative Neurological Diseases, Kodaira, Tokyo, Japan.
- 713 Tohoku University, Graduate School of Life Sciences, Department of Integrative Life Sciences, Sendai, Miyagi, Japan.
- 714 Goethe-University Frankfurt, Institute for Experimental Cancer Research in Pediatrics, Frankfurt, Germany.
- 715 Monash University, Cancer Program, Biomedicine Discovery Institute and Department of Anatomy & Developmental Biology, VIC, Australia; Peter MacCallum Cancer Centre, Prostate Cancer Translational Research Laboratory, Melbourne, Victoria, Australia; University of Melbourne, Sir Peter MacCallum Department of Oncology, Parkville, VIC, Australia.
- 716 Juntendo University Graduate School of Medicine, Department of Neurology, Bunkyo-ku, Tokyo Japan.
- 717 The University of Chicago, Department of Microbiology, Chicago, IL, USA.
- 718 University of Gdansk, Department of Molecular Biology, Gdansk, Poland.
- 719 New York University - Abu Dhabi, Saadiyat Island Campus, Division of Science (Biology), Cell Death Signaling Laboratory, Abu Dhabi, UAE.
- 720 University of Oxford, Sir William Dunn School of Pathology, Oxford, UK.
- 721 Universidad Castilla La Mancha, Facultad de Farmacia, Área Tecnología Farmacéutica, Albacete, Spain.
- 722 Beckman Research Institute at City of Hope, Department of Systems Biology, Monrovia, CA, USA.
- 723 Weill Cornell Medical College, Department of Radiation Oncology, New York, NY, USA; Sandra and Edward Meyer Cancer Center, New York, NY, USA; Caryl and Israel Englander Institute for Precision Medicine, New York, NY, USA; Yale School of Medicine, Department of Dermatology, New Haven, CT, USA; Université de Paris, Paris, France.
- 724 Sorbonne Université, Developmental Biology Laboratory, CNRS, Institut de Biologie Paris Seine, IBPS, UMR7622, Paris, France.
- 725 University of Edinburgh, Cancer Research UK Edinburgh Centre, Institute of Genetics and Molecular Medicine, Edinburgh, UK.
- 726 University of Texas MD Anderson Cancer Center, Department of Experimental Radiation Oncology, Houston, TX, USA.
- 727 MRC Protein Phosphorylation and Ubiquitylation Unit, School of Life Sciences, University of Dundee, UK.
- 728 Fourth Military Medical University, School of Aerospace Medicine, Xi'an, China.
- 729 Chinese Academy of Sciences, Institute of Zoology, State Key Laboratory of Stem cell and Reproductive Biology, Beijing, China.
- 730 Harbin Institute of Technology, the HIT Center for Life Sciences, School of Life Sciences and Technology, Harbin, China.
- 731 South China University of Technology, School of Medicine and Institute for Life Sciences, GuangZhou, China.
- 732 University of Pittsburgh School of Medicine, Hillman Cancer Center and Department of Microbiology and Molecular Genetics, Pittsburgh, PA, USA.
- 733 University of Pittsburgh School of Medicine, Department of Surgery, Pittsburgh, PA, USA.
- 734 Chinese Academy of Sciences Center for Excellence in Molecular and Cellular Sciences, Shanghai Institute of Biochemistry and Cell Biology, Shanghai, China.
- 735 Universitat de Lleida, Department of Experimental Medicine, IRBLleida, Lleida, Spain.
- 736 Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Departamento de Microbiología, Inmunología y Biotecnología, Cátedra de Inmunología, Buenos Aires, Argentina; Universidad de Buenos Aires, CONICET, Instituto de Estudios de la Inmunidad Humoral (IDEHU), Buenos Aires, Argentina.
- 737 Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Instituto de Química Biológica de la Facultad de Ciencias Exactas y Naturales (IQUIBICEN), Buenos Aires, Argentina.
- 738 National Center for Biotechnology (CNB)-CSIC, Laboratory of Intracellular Bacterial Pathogens, Madrid, Spain.
- 739 Universidad Autónoma de Madrid, Facultad de Medicina, Departamento de Anatomía, Histología y Neurociencia, Madrid, Spain.
- 740 Universidad Autonoma de Nuevo Leon, School of Medicine, Department of Histology, Monterrey, Nuevo Leon, Mexico.
- 741 Universidad de Salamanca, Institute of Functional Biology and Genomics (IBFG), Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, Spain.
- 742 Department of Cell Biology and Histology, Faculty of Biology, University of Murcia, IMIB-Arrixaca, Murcia, Spain.
- 743 Department of Cell Death and Proliferation, Institute of Biomedical Research of Barcelona (IIBB- CSIC); Liver Unit, Hospital Clinic i Provincial de Barcelona, IDIBAPS, CIBEREHD, Barcelona, Spain.
- 744 Cajal Institute/CSIC and CIBERNED, ISCIII, Madrid, Spain.
- 745 KU Leuven, Department of Cellular and Molecular Medicine, Leuven, Belgium.
- 746 Centro de Biología Molecular Severo Ochoa (CSIC-UAM), Madrid, Spain.
- 747 Sapienza University of Rome, Department of Experimental Medicine, Rome, Italy.
- 748 Tulane University, Department of Microbiology and Immunology, New Orleans, LA, USA.
- 749 University of Bonn, Clinical Centre, Department of Psychiatry, Neurohomeostasis Group, Bonn, Germany.
- 750 Zhejiang University School of Medicine,Women's Hospital,Hangzhou, Zhejiang, China.
- 751 University of Milano, Department of Biomedical Sciences for Health, Milan, Italy.
- 752 Institut de Biologie Moléculaire des Plantes, Centre National de la Recherche Scientifique, Unité Propre de Recherche 2357, Conventionné avec l'Université de Strasbourg, Strasbourg, France.
- 753 University Clinic Freiburg, Institute for Microbiology and Hygiene, University of Freiburg - Faculty of Medicine, Freiburg, Germany.
- 754 Mortimer B. Zuckerman Mind Brain and Behavior Institute, Columbia University, New York, NY, USA.
- 755 Institute for Animal Developmental and Molecular Biology, Heinrich-Heine University Düsseldorf, Düsseldorf, Germany.
- 756 Virginia Commonwealth University, Departments of Pharmacology, Toxicology and Medicine and Massey Cancer Center, Richmond, VA, USA.
- 757 Kerman University of Medical Sciences, Institute of Neuropharmacology, Neuroscience Research Center, Kerman, Iran.
- 758 Autophagy Research Center, Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran.
- 759 University of Torino, Department of Molecular Biotechnology and Health Sciences, Torino, Italy.
- 760 KU Leuven, Department of Public Health and Primary Care, Centre Environment & Health, Leuven, Belgium.
- 761 University of Sussex, School of Life Sciences, Department of Biochemistry and Biomedicine, Brighton, UK.
- 762 Sapienza University of Rome, Department of Anatomy, Histology, Forensic Medicine and Orthopedics, Rome, Italy.
- 763 Democritus University of Thrace, Medical School, Department of Pathology, Alexandroupolis, Greece.
- 764 Weill Cornell Medicine, Brain and Mind Research Institute, Burke Neurological Institute, White Plains, NY, USA.
- 765 University of Manitoba, Departments of Biochemistry and Medical Genetics, Winnipeg, Canada.
- 766 Department of Fundamental Neurosciences, University of Lausanne, Lausanne, Switzerland.
- 767 Clinic of Neonatology, Department of Women, Mother and Child, University Hospital Center of Vaud (CHUV), Lausanne, Switzerland.
- 768 National Institutes of Health, National Institutes of Neurological Disorders and Stroke (NINDS), Bethesda, MD, USA.
- 769 University of Ferrara, Department of Morphology, Surgery and Experimental Medicine, Ferrara, Italy.
- 770 University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Faculty of Medicine, Coimbra, Portugal; University of Coimbra, Center for Innovative Biomedicine and Biotechnology, Coimbra, Portugal.
- 771 University of Toronto, Department of Laboratory Medicine and Pathobiology, Toronto, Ontario, Canada.
- 772 University of California, Davis and MIND Institute, School of Veterinary Medicine, Dept. Molecular Biosciences, Davis, CA, USA.
- 773 INSERM, UMR1037 CRCT, Toulouse, France; Université Toulouse III-Paul Sabatier, UMR1037 CRCT, Toulouse, France; CNRS, ERL5294 CRCT, Toulouse, France.
- 774 University of Cologne, Faculty of Medicine and University Hospital Cologne, Institute for Medical Microbiology, Immunology and Hygiene, Cologne, Germany.
- 775 University of Seville, Department of Genetics, Seville, Spain.
- 776 Ludwig Institute for Cancer Research; University of California San Diego, Department of Cellular and Molecular Medicine, La Jolla, CA, USA.
- 777 Medical University of Warsaw, Department of Immunology, Warsaw, Poland.
- 778 University of Auckland, School of Biological Sciences, Auckland, New Zealand.
- 779 Luxembourg Institute of Health, Department of Oncology, NorLux Neuro-Oncology Laboratory, Luxembourg, Luxembourg.
- 780 Center of Toxins, Immune-response and Cell Signaling (CeTICS) and Laboratório Especial de Ciclo Celular (LECC), Instituto Butantan, São Paulo, Brazil.
- 781 University of Groningen, University Medical Center of Groningen, Department of Biomedical Sciences of Cells and Systems, Groningen, The Netherlands.
- 782 Leiden University Medical Center, Department of Cell and Chemical Biology and Oncode Institute, Leiden, The Netherlands.
- 783 Shanghai Jiao Tong University, School of Life Sciences and Biotechnology, Shanghai, China.
- 784 University of León, Institute of Biomedicine (IBIOMED) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), León, Spain.
- 785 University of La Laguna, Institute of Biomedical Technologies, Department of Basic Medical Sciences, La Laguna, Tenerife, Spain.
- 786 University of Alberta, Department of Biochemistry, Edmonton, Alberta, Canada.
- 787 University of Alabama at Birmingham, Department of Optometry and Vision Science, Birmingham, AL, USA.
- 788 The Henry M. Jackson Foundation, Inc. Bethesda, MD, USA.
- 789 Technische Universität München, Klinikum rechts der Isar, Comprehensive Cancer Center München, München, Germany.
- 790 Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Instituto de Química Biológica Ciencias Exactas y Naturales (IQUIBICEN), Facultad de Ciencias Exactas y Naturales, Departamento de Química Biológica, Laboratorio de Disfunción Celular en Enfermedades Neurodegenerativas y Nanomedicina, Buenos Aires, Argentina.
- 791 BC Cancer, Michael Smith Genome Sciences Centre, Vancouver, BC, Canada.
- 792 Harvard Medical School, Department of Dermatology, Cutaneous Biology Research Center Massachusetts General Hospital, Boston, MA, USA.
- 793 Consejo Superior de Investigaciones Científicas, Instituto de Bioquímica Vegetal y Fotosíntesis (CSIC-IBVF), Sevilla, Spain.
- 794 Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
- 795 Tel Aviv University, Sackler Faculty of Medicine and Sagol School of Neuroscience, Department of Human Molecular Genetics and Biochemistry, Tel Aviv, Israel.
- 796 Max Planck Institute for Biology of Ageing, Cologne, Germany; University of Cologne, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany.
- 797 Jena University Hospital, Department of Anesthesiology and Intensive Care Medicine, Center for Molecular Biomedicine (CMB) and Center for Sepsis Control and Care (CSCC), Jena, Germany.
- 798 Weill Cornell Medicine, Feil Family Brain and Mind Research Institute, New York, NY, USA.
- 799 University of Texas MD Anderson Cancer Center, Department of Cancer Systems Imaging, Houston, TX, USA.
- 800 University of the West of England, Department of Applied Sciences, Bristol, UK.
- 801 Flinders University, College of Medicine and Public Health, Adelaide, Australia.
- 802 University of Alabama at Birmingham,Center for Neurodegeneration and Experimental Therapeutics, Department of Neurology, Birmingham, AL, USA.
- 803 Boston University, Departments of Biomedical Engineering, Chemistry, and Medicine, Boston, MA, USA.
- 804 Université de Strasbourg/CNRS UPR 3572 Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France.
- 805 University of Iowa, Departments of Pediatrics and Microbiology, Iowa City, IA, USA.
- 806 Max Planck Institute for Biology of Ageing, Cologne, Germany.
- 807 German Institute of Human Nutrition, Department of Molecular Toxicology, Nuthetal, Germany.
- 808 Lanzhou University, School of Public Health, Lanzhou, Gansu, China.
- 809 Case Western Reserve University, Comprehensive Cancer Center, Cleveland, OH, USA.
- 810 University of Salento, Department of Biological and Environmental Sciences and Technologies, Lecce, Italy.
- 811 Research Center Principe Felipe, Cellular Pathology Laboratory, Valencia, Spain.
- 812 Johns Hopkins University School of Medicine, Department of Neuroscience, Baltimore, MD, USA.
- 813 School of Pharmacy, Complutense University, Madrid, Spain; Centro de Investigación Biomédica en Red (CIBER) de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Madrid, Spain.
- 814 Dalhousie University, Faculty of Medicine, Departments of Pathology, Biology, and Microbiology & Immunology, Halifax, NS, Canada.
- 815 David Geffen School of Medicine at UCLA, Department of Medicine, Los Angeles, CA, USA.
- 816 KU Leuven, Clinical Division and Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, Leuven, Belgium.
- 817 Justus-Liebig University, Department of Internal Medicine, Giessen, Germany.
- 818 Center for Molecular Medicine, Maine Medical Center Research Institute, Scarborough, ME, USA.
- 819 Tongji University School of Medicine, Shanghai Tenth People's Hospital, Department of Gastroenterology, Shanghai, China.
- 820 The University of Sheffield, Department of Biomedical Science, Firth Court, Western Bank, Sheffield, UK.
- 821 University of Virginia, School of Medicine, Department of Surgery, Charlottesville, VA, USA.
- 822 University of California, Los Angeles, Department of Neurology, Molecular and Medical Pharmacology, David Geffen School of Medicine, Los Angeles, CA, USA.
- 823 Institute of Microbial Technology, Department of Molecular Biology, Chandigarh, India.
- 824 CSIR-Central Drug Research Institute, Lucknow, India.
- 825 University of Oslo, Institute of Basic Medical Sciences, Oslo, Norway.
- 826 Deakin University, School of Medicine, Faculty of Health, Victoria, Australia.
- 827 Macquarie University, Faculty of Medicine, Health and Human Sciences, New South Wales, Australia.
- 828 University of California, San Diego, Skaggs School of Pharmacy and Pharmaceutical Sciences, La Jolla, CA, USA.
- 829 Medical College of Wisconsin, Department of Medicine and Cardiovascular Center, Milwaukee, WI, USA.
- 830 ICAR-Indian Veterinary Research Institute, FMD VP Laboratory, Bengaluru, India.
- 831 University of Eastern Finland, A.I. Virtanen Institute for Molecular Sciences, Kuopio, Finland.
- 832 Brandeis University, Department of Biology and Rosenstiel Basic Medical Sciences Research Center, Waltham, MA, USA.
- 833 University of Gdansk, Department of Medical Biology and Genetics, Gdansk, Poland.
- 834 Tokai University School of Medicine, Department of Molecular Life Sciences, Isehara, Kanagawa, Japan.
- 835 Swedish Agricultural University (SLU), Department of Plant Biology, Uppsala, Sweden.
- 836 Hasselt University, Biomedical Research Institute, Diepenbeek, Belgium.
- 837 University of Surrey, School of Bioscience and Medicine, Department of Microbial Sciences, Stag Hill Campus, Guildford, Surrey, UK.
- 838 Barts Cancer Institute, Queen Mary University of London, Centre for Biotherapeutics and Biomarkers, London, UK.
- 839 Johns Hopkins University Bloomberg School of Public Health, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Baltimore, MD, USA.
- 840 Goethe University, Institute for Molecular Biosciences, Frankfurt/Main, Germany.
- 841 Osaka University, Graduate School of Medicine, Department of Genetics, Suita, Osaka, Japan.
- 842 Zhejiang University, Sir Run Run Shaw Hospital, College of Medicine, Department of Medical Oncology, Zhejiang, Hangzhou, China.
- 843 Sanford Burnham Prebys Medical Discovery Institute, Program of Development, Aging and Regeneration, La Jolla, CA, USA.
- 844 University of Michigan, Department of Biological Chemistry, Ann Arbor, MI, USA.
- 845 Third Department of Internal Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
- 846 Department of Neurophysiology, Institute for Biological Research "Siniša Stanković" - National Institute of Republic of Serbia, University of Belgrade, Belgrade, Serbia.
- 847 University of California, Davis, School of Medicine, Department of Pharmacology, Davis, CA, USA.
- 848 University of Toronto, Department of Medicine, Toronto, Ontario, Canada.
- 849 Monash University, School of Clinical Sciences at Monash Health, Centre for Inflammatory Diseases, Rheumatology Group, Clayton, Victoria, Australia.
- 850 Tohoku University Graduate School of Medicine, Division of Neurology, Department of Neuroscience & Sensory Organs, Sendai, Japan.
- 851 University of Malaya, Institute of Biological Sciences (Genetics), Kuala Lumpur, Malaysia.
- 852 Washington University School of Medicine, Division of Pulmonary and Critical Care Medicine, St. Louis, MO, USA.
- 853 Leibniz Forschungsinstitut für Molekulare Pharmakologie (FMP), Department of Molecular Pharmacology and Cell Biology, Berlin, Germany.
- 854 California Institute of Technology, Division of Biology and Biological Engineering, Pasadena, CA, USA.
- 855 University of Massachusetts Medical School, Molecular, Cell and Cancer Biology, Worcester, MA, USA.
- 856 Bulgarian Academy of Sciences, Institute of Biology and Immunology of Reproduction Acad. Kiril Bratanov, Sofia, Bulgaria.
- 857 University of Minnesota, Department of Genetics, Cell Biology and Development, Minneapolis, MN, USA.
- 858 Northwestern University, Feinberg School of Medicine, Department of Cell and Developmental Biology, Chicago, IL, USA.
- 859 Sichuan University, West China School of Pharmacy, Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Chengdu, China.
- 860 Jinan University, College of Pharmacy, Guangzhou, China.
- 861 Department of Immunology, Duke University Medical Center, Durham, NC, USA.
- 862 University of Chicago, Department of Medicine, Section of Dermatology, Chicago, IL, USA.
- 863 D'Youville College, School of Pharmacy, Department of Pharmaceutical, Social and Administrative Sciences, Buffalo, NY, USA.
- 864 University of Innsbruck, Institute of Biochemistry and Center for Molecular Biosciences Innsbruck, Innsbruck, Austria; University Medical Center Groningen, University of Groningen, Section Systems Medicine of Metabolism and Signaling, Laboratory of Pediatrics, Groningen, The Netherlands.
- 865 National Institutes of Health, National Eye Institute, Ophthalmic Genetics and Visual Function Branch, Ophthalmic Molecular Genetics Section, Bethesda, MD, USA.
- 866 University of Glasgow, Wolfson Wohl Cancer Research Centre, Institute of Cancer Sciences, Glasgow, UK.
- 867 University College London, Cancer Institute, London, UK.
- 868 Universidade Federal de São Carlos, Department of Genetics and Evolution, São Carlos (SP), Brazil.
- 869 Instituto de Investigación Sanitaria de Navarra (IdISNA), Fundación Miguel Servet, Biomedical Research Center of Navarre-Navarrabiomed, Immunomodulation Group, Pamplona, Spain.
- 870 Université de Bordeaux, Institut des Maladies Neurodégénératives (IMN), CNRS UMR 5293, Bordeaux, France.
- 871 University of Barcelona, Barcelona Hepatic Hemodynamic Unit, Liver Unit, Hospital Clinic, Hospital Clínic-Institut d'Investigacions Biomèdiques (IDIBAPS), Centro de Investigación Biomédica Red de enfermedades Hepáticas y Digestivas (CIBERehd), Barcelona, Spain.
- 872 INRA, UR1037 Laboratory of Fish Physiology and Genomics, Campus de Beaulieu, Rennes, France; Hunan Normal University, College of Life Sciences, State Key Laboratory of Developmental Biology of Freshwater Fish, Changsha, Hunan, China.
- 873 University of Lleida, Department of Basic Medical Sciences, IRBLleida, Lleida, Spain.
- 874 Universidad del País Vasco, Instituto Biofisika (CSIC, UPV/EHU) and Departamento de Bioquímica y Biología Molecular, Bilbao, Spain.
- 875 Centenary Institute, The University of Sydney, Newtown, New South Wales, Australia; Faculty of Medicine and Health, The University of Sydney, Newtown, New South Wales, Australia.
- 876 University of Chile, Biomedical Neuroscience Institute, Faculty of Medicine, Santiago, Chile; FONDAP Center for Geroscience Brain Health and Metabolism, Santiago, Chile; University of Chile, Institute of Biomedical Science, Program of Cellular and Molecular Biology, Santiago, Chile; Buck Institute for Research on Aging, Novato, CA, USA.
- 877 University of Bern, Institute of Cell Biology, Bern, Switzerland.
- 878 Kyushu University, Depatment of Bioscience and Biotechnology, Nishi-ku, Fukuoka, Japan.
- 879 UT Southwestern Medical Center, Departments of Medicine (Cardiology) and Molecular Biology, Dallas, TX, USA.
- 880 Theoretical Division, Los Alamos National Laboratory, Los Alamos, NM, USA.
- 881 University of Waterloo, School of Pharmacy, Kitchener, ON, Canada.
- 882 University of Hong Kong, Department of Medicine, Division of Neurology, Pokfulam, Hong Kong, China.
- 883 National University of Singapore, Department of Physiology, Singapore.
- 884 University of North Carolina at Chapel Hill, Department of Pharmacology, Chapel Hill, NC, USA.
- 885 Yale University, Department of Molecular Biophysics and Biochemistry, New Haven, CT, USA.
- 886 Swedish University of Agricultural Sciences (SLU) and Linnean Center for Plant Biology, Uppsala BioCenter, Department of Plant Biology, Uppsala, Sweden.
- 887 University Côte d'Azur, FHU OncoAge, Department of Pathology, Nice, France.
- 888 German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Department of Molecular Toxicology, Nuthetal, Germany.
- 889 University of Helsinki, Medicum, Biochemistry and Developmental Biology, Helsinki, Finland.
- 890 University of Birmingham, College of Medical and Dental Sciences, Institute of Inflammation and Ageing, Birmingham, UK.
- 891 Kyungpook National University, School of Medicine, Department of Physiology, Daegu, Korea.
- 892 Kaohsiung Medical University, Graduate Institute of Medicine & Department of Biochemistry, Faculty of Medicine, College of Medicine, Kaohsiung, Taiwan.
- 893 University of Cologne, Institute for Genetics and CECAD Research Center, Cologne, Germany.
- 894 European Molecular Biology Laboratory, Heidelberg, Germany.
- 895 University of Texas Southwestern Medical Center, Department of Internal Medicine, Dallas, TX, USA.
- 896 Chung Shan Medical University, Institute of Medicine, Taiwan.
- 897 National Chiao-Tung University, Department of Applied Chemistry and Institute of Molecular Science, Hsinchu, Taiwan.
- 898 University of Plymouth, Faculty of Health: Medicine, Dentistry and Human Sciences, Peninsula Dental School, Plymouth, Devon, UK.
- 899 Anhui University of Science and Technology, Department of Medical Immunology, Huainan, China.
- 900 Soochow University, Institute of Neuroscience, Suzhou, Jiangsu Province, China.
- 901 University of Texas Southwestern Medical Center, Department of Internal Medicine, Charles and Jane Center for Mineral Metabolism and Clinical Research, Dallas, TX, USA.
- 902 Southern Medical University, Shenzhen Hospital, Department of Gastroenterology, Shenzhen, Guangdong.
- 903 National Tsing Hua University, Department of Chemical Engineering, and Frontier Research Center on Fundamental and Applied Sciences of Matters, Hsinchu, Taiwan.
- 904 Northwest A&F University, College of Veterinary Medicine, Shaanxi Centre of Stem Cells Engineering & Technology, Yangling, Shaanxi, China.
- 905 Shanghai Jiao Tong University School of Medicine, Shanghai General Hospital, Department of Orthopedics, Shanghai, China; Shanghai Bone Tumor Institution, Shanghai, China.
- 906 State University of New York, Downstate Health Sciences University, Department of Surgery and Cell Biology, Brooklyn, NY, USA.
- 907 Sichuan University, and Collaborative Innovation Center for Biotherapy, West China Hospital, West China School of Basic Medical Sciences & Forensic Medicine, and State Key Laboratory of Biotherapy and Cancer Center, Chengdu, China.
- 908 New York University School of Medicine, Department of Environmental Medicine, Urology, Biochemistry and Molecular Pharmacology, New York, NY, USA.
- 909 Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Immunology & Department of Immunology and Microbiology, Shanghai, China.
- 910 University of Sydney, Kolling Institute, Renal Research Lab, Sydney, New South Wales, Australia.
- 911 Wenzhou Medical University, School of Laboratory Medicine and Life Sciences, Wenzhou, Zhejiang Province, China.
- 912 Huazhong University of Science and Technology, Tongji Medical College, School of Pharmacy, Wuhan, Hubei, China.
- 913 Chongqing University, School of Medicine, Center for Neurointelligence, Chongqing, China.
- 914 The Second Affiliated Hospital of Xi'an Jiaotong University, Department of Radiation Oncology, Xi'an, Shaan Xi, China.
- 915 Zhejiang University School of Medicine, The First Affiliated Hospital, Zhejiang Provincial Key Laboratory of Pancreatic Disease, Hangzhou, Zhejiang, China.
- 916 III. Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
- 917 Medical University of Vienna, Department of Pathology, Vienna, Austria.
- 918 University Hospital Jena, Institute of Human Genetics, Jena, Thuringia, Germany.
- 919 University of Otago, School of Biomedical Sciences and Brain Health Research Centre, Department of Biochemistry, Dunedin, New Zealand.
- 920 University of Bern, Institute of Pathology, Bern, Switzerland; TRANSAUTOPHAGY: European network for multidisciplinary research and translation of autophagy knowledge, COST Action CA15138.
- 921 Max Planck Institute of Biophysics, Department of Theoretical Biophysics, Frankfurt am Main, Germany.
- 922 University of California, Berkeley, Department of Molecular and Cell Biology, Berkeley, CA, USA.
- 923 West Virginia University, School of Medicine, Department of Physiology and Pharmacology, Morgantown, WV, USA.
- 924 Durham University, Department of Biosciences, Durham, UK.
- 925 University of Indonesia, Faculty of Medicine, Department of Obstetric and Gynecology, Jakarta, Indonesia.
- 926 Yonsei University, Department of Biotechnology, Seodaemun-gu, Seoul, Korea.
- 927 The University of Chicago, Department of Pathology, Chicago, IL, USA; present address: VIR Biotechnology, San Francisco, CA, USA.
- 928 University of Messina, Department of Human Pathology in Adult and Developmental Age "Gaetano Barresi", Section of Pathology, Messina, Italy.
- 929 Kyushu University, Medical Institute of Bioregulation (MIB), Fukuoka, Japan.
- 930 Osaka International Cancer Institute, Department of Molecular and Cellular Biology, Osaka, Japan.
- 931 Juntendo University Graduate School of Medicine, Department of Research for Parkinson's Disease, Tokyo, Japan.
- 932 University of Modena and Reggio Emilia, Department of Life Sciences, Modena, Italy.
- 933 Juntendo University, Juntendo University Graduate School of Medicine, Division for Development of Autophagy Modulating Drugs, Tokyo, Japan.
- 934 University of North Texas Health Science Center, North Texas Eye Research Institute and Department of Pharmaceutical Sciences, Fort Worth, TX, USA.
- 935 University of Michigan, Life Sciences Institute, Department of Molecular and Integrative Physiology, Ann Arbor, MI, USA.
- 936 Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR 7258, Aix-Marseille Université and Institut Paoli-Calmettes, Parc Scientifique et Technologique de Luminy, Marseille, France.
- 937 University of Massachusetts Medical School, Department of Microbiology and Physiological Systems, Worcester, MA, USA.
- 938 National University of Córdoba, Clinical Biochemistry Department, CIBICI-CONICET, Córdoba, Argentina.
- 939 Stanford University School of Medicine, Department of Pathology, Stanford, CA, USA.
- 940 Akita University Graduate School of Medicine, Department of Ophthalmology, Akita, Japan.
- 941 Vascular Biology Center, Medical College of Georgia, Augusta University, Augusta, GA, USA; South China University of Technology, Guangzhou First People's Hospital, School of Medicine and Institutes for Life Sciences, Guangzhou, Guangdong, China.
- 942 University of Copenhagen, Copenhagen Biocentre, Faculty of Health and Medical Sciences, Biotech Research & Innovation Centre (BRIC), Neuroinflammation Unit, Copenhagen N, Denmark.
- 943 Chiba University, Department of Biology, Chiba, Japan.
- 944 Keio University School of Medicine, Department of Neurology, Tokyo, Japan.
- 945 University College London, Department of Neuroscience, Physiology and Pharmacology, London, UK.
- 946 Institute for Research in Biomedicine (IRB Barcelona), Barcelona, Spain; Universitat de Barcelona, Departament de Bioquímica I Biomedicina Molecular, Barcelona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
- 947 Barcelona Institute of Science and Technology (BIST), Institute for Research in Biomedicine (IRB Barcelona), Barcelona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas, Barcelona, Spain; Universitat de Barcelona, Facultat de Biologia, Departament de Bioquimica i Biomedicina Molecular, Barcelona, Spain.
- 948 Hampton University, School of Pharmacy, Department of Pharmaceutical Sciences, Hampton, VA, USA.
- 949 Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas, Universidad Autónoma de Madrid, Madrid, Spain; Department of Cell Biology and Immunology, Campus de Cantoblanco, Madrid, Spain.
- 950 RIKEN, Center for Sustainable Resource Science, Wako, Saitama, Japan.
- 951 Danish Cancer Society Research Center, Center for Autophagy, Recycling and Disease, Cell Death and Metabolism Unit, Copenhagen, Denmark; University of Copenhagen, Department of Cellular and Molecular Medicine, Copenhagen, Denmark.
- 952 University of Technology, Department of applied science, Division of Biotechnology, Baghdad, Iraq.
- 953 University of Maryland School of Medicine, Department of Microbiology and Immunology, Baltimore, MD, USA.
- 954 Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Unidad de Bioquimica, Mexico City, Mexico.
- 955 University of Warwick, School of Life Sciences, Coventry, UK.
- 956 Université Paris-Saclay, INSERM ,UMR-S 1193, Châtenay-Malabry, France.
- 957 Lewis Katz School of Medicine at Temple University, Center for Translational Medicine, Philadelphia, PA, USA.
- 958 Houston Methodist Research Institute, Weill-Cornell Medicine, Houston, TX, USA.
- 959 Delft University of Technology, Kavli Institute of Nanoscience, Department of Bionanoscience, Delft, The Netherlands.
- 960 Lund University, Department of Experimental Medical Science, Laboratory of Molecular Neurogenetics, Wallenberg Neuroscience Center and Lund Stem Cell Center, Lund, Sweden.
- 961 Luxembourg Institute of Health, Tumor Immunotherapy and Microenvironment (TIME) group, Department of Oncology, Luxembourg City, Luxembourg.
- 962 Cancer Drug Resistance and Stem Cell Program, University of Sydney, NSW, Australia.
- 963 University Hospital of Regensburg, Institute of Clinical Microbiology and Hygiene, Regensburg , Germany.
- 964 Polish Academy of Sciences, Mossakowski Medical Research Centre, Laboratory of Ischemic and Neurodegenerative Brain Research, Warsaw, Poland.
- 965 Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan; Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
- 966 Université de Sherbrooke, Département d'immunologie et de biologie cellulaire, Sherbrooke, Québec, Canada.
- 967 UMR7242, CNRS, Université de Strasbourg, Strasbourg, France.
- 968 Institute of Experimental Medicine CAS, Department of Neuroregeneration, Prague, Czech Republic.
- 969 Albert Einstein College of Medicine, Department of Developmental and Molecular Biology and Department of Genetics, New York, NY, USA.
- 970 University of Copenhagen, Department of Nutrition, Exercise and Sports, Section of Molecular Physiology, Copenhagen, Denmark.
- 971 Aarhus University Hospital, Steno Diabetes Center Aarhus, Aarhus, Denmark.
- 972 University of Texas Southwestern Medical Center, Department of Molecular Biology, Dallas, TX, USA.
- 973 The First Affiliated Hospital of Nanjing Medical University, Department of Neurosurgery, Nanjing, Jiangsu, China.
- 974 Shanghai University of Traditional Chinese Medicine, Longhua Hospital, Cancer Institute, Shanghai, China.
- 975 Purdue University, Department of Nutrition Science, West Lafayette, IN, USA.
- 976 Karolinska Institutet, Department of Neurobiology, Care Sciences and Society, Division of Neurogeriatrics, Solna, Stockholm, Sweden.
- 977 The First Hospital of Jilin University, Department of Ear, Nose and Throat, Changchun, Jilin, China.
- 978 Nanjing Medical University, Nanjing First Hospital, Department of Neurology, Nanjing, China.
- 979 University of Pittsburgh, Department of Pharmacology and Chemical Biology, Pittsburgh, PA, USA.
- 980 King's College London, Institute of Psychiatry, Psychology & Neuroscience, Department of Basic and Clinical Neuroscience, Maurice Wohl Clinical Neuroscience Institute, London, UK.
- 981 Wonkwang Univesity, Department of Biological Sciences, Iksan, Chunbuk, Korea.
- 982 The First Hospital of Jilin University, Cancer Center, Department of Hematology, Changchun, Jilin, China.
- 983 ZhejiangUniversity, Sir Run Run Shaw Hospital, School of Medicine, Key Lab of Biotherapy in Zhejiang Province, Hangzhou, China.
- 984 Xi'an Jiaotong University, School of Medicine, the First Affiliated Hospital, Department of Nephrology, Xi'an City, Shaanxi Province, China.
- 985 Huazhong University of Science and Technology, Liyuan hospital of Tongji Medical College, Department of Endocrinology, Wuhan, Hubei, China.
- 986 Chungnam National University School of Medicine, Infection Control Convergence Research Center, Daejeon, Korea.
- 987 Cancer Research Center of Toulouse (CRCT), INSERM U1037, CNRS ERL5294, University of Toulouse, Toulouse, France.
- 988 University of Rochester, Department of Anesthesiology and Perioperative Medicine, Rochester, NY, USA.
- 989 University of Sheffield, Department of Infection, Immunity and Cardiovascular Disease and the Bateson Centre, Sheffield, UK.
- 990 University of Helsinki, Institute of Biotechnology, Cell and Tissue Dynamics Programme and Electron Microscopy Unit, Helsinki, Finland.
- 991 Indian Institute of Science, Centre for BioSystems Science and Engineering, Bangalore, India.
- 992 Radboud University Medical Centre, Department of Internal Medicine, Nijmegen, The Netherlands.
- 993 Universidad Castilla La Mancha, Departmento Ciencias Medicas, Albacete, Spain.
- 994 Fudan University School of Pharmacy, Department of Biological Medicines, Shanghai, China.
- 995 The University of Suwon, Department of Health Science, Hwaseong, Gyeonggi, Korea.
- 996 Stony Brook University, Department of Pathology, Stony Brook, NY, USA.
- 997 Instituto Nacional de Enfermedades Respiratorias Isamel Cosío Villegas, Departamento de Investigación en Microbiología, Ciudad de México, México.
- 998 Gwangju Institute of Science and Technology, School of Life Sciences, Gwangju, South Korea.
- 999 Korea Food Research Institute, Research Group of Natural Material and Metabolism, Jeollabuk-do, Korea.
- 1000 Ewha Womans University, Department of Biochemistry, College of Medicine, Seoul, Korea.
- 1001 Seoul National University, School of Biological Science, Seoul, Korea.
- 1002 Evelina Children's Hospital, Guy's & St Thomas' NHS Foundation Trust, Children's Neuroscience Centre, London, UK; King's College London, Muscle Signalling Section, Randall Division of Cell and Molecular Biophysics, London, UK; King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), Department of Basic and Clinical Neuroscience, London, UK.
- 1003 Memorial Sloan Kettering Cancer Center, New York, NY, USA.
- 1004 University of Ulm, Department of Internal Medicine II, Molecular Cardiology, Ulm, Germany.
- 1005 University of Eastern Finland and Kuopio University Hospital, Department of Ophthalmology, Kuopio, Finland.
- 1006 University Tartu, Department of Pharmacology, Tartu, Estonia.
- 1007 University Medical Center Gottingen, Department of Experimental Neurodegeneration, Gottingen, Germany.
- 1008 University of Michigan, Kellogg Eye Center, Department of Ophthalmology and Visual Sciences, Ann Arbor, MI, USA.
- 1009 University of California, San Francisco UCSF Diabetes Center, Department of Cell and Tissue Biology, San Francisco, CA, USA.
- 1010 University of Kansas Medical Center, Department of Microbiology, Molecular Genetics and Immunology, Kansas City, KS, USA.
- 1011 Regional Centre for Biotechnology, NCR Biotech Science Cluster, Faridabad, India.
- 1012 National Research Council of Italy (CNR), Neuroscience Institute, Padua, Italy.
- 1013 European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI), Wellcome Genome Campus, Hinxton, Cambridge, UK.
- 1014 Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland.
- 1015 Karolinska Institutet, Department of Oncology-Pathology, Stockholm, Sweden.
- 1016 Gifu University Graduate School of Medicine, Department of Cardioligy, Gifu, Japan.
- 1017 Shimane University Faculty of Medicine, Internal Medicine 1, Izumo, Shimane, Japan.
- 1018 Seoul National University, School of Biological Sciences, Seoul, Korea.
- 1019 UT Southwestern Medical Center, Department of Surgery, Dallas, Texas, USA.
- 1020 Chungbuk National University, Department of Biology Education, Seowon-Gu, Cheongju, Chungbuk, Korea.
- 1021 CAESAR Research Center, Bonn, Germany.
- 1022 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany.
- 1023 Niigata University Graduate School of Medical and Dental Sciences, Department of Cellular Physiology, Niigata, Japan.
- 1024 Department of Biomedical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA, USA.
- 1025 Florida Atlantic University, Charles E. Schmidt College of Medicine, Department of Biomedical Science, Boca Raton, FL, USA.
- 1026 Semmelweis University, Budapest, Hungary.
- 1027 National and Kapodistrian University of Athens, Medical School, Division of Molecular Oncology, Department of Biological Chemistry, Athens, Greece.
- 1028 University of Minnesota, Institute for Translational Neuroscience, Department of Neuroscience, Minneapolis, MN, USA.
- 1029 Jadavpur University, Department of Life Science and Biotechnology, Kolkata, India.
- 1030 University of Otago, Department of Physiology-HeartOtago, Dunedin, Otago, New Zealand.
- 1031 Hokkaido University, Department of Rheumatology, Endocrinology and Nephrology, Sapporo, Japan.
- 1032 Max Planck Institute for Infection Biology, Berlin, Germany.
- 1033 University of Eastern Finland, Faculty of Health Sciences, School of Pharmacy, Kuopio, Finland.
- 1034 University of Arkansas for Medical Sciences, Little Rock, AR, USA.
- 1035 Doshisha Women's College of Liberal Arts, Faculty of Pharmaceutical Sciences, Kyotanabe, Kyoto, Japan.
- 1036 Commonwealth Scientific and Industrial Research Organization (CSIRO), Agriculture and Food, Brisbane, Queensland, Australia.
- 1037 Chang Gung University, Department of Biochemistry and Molecular Biology, Taoyuan, Taiwan, Republic of China.
- 1038 Flinders University, Flinders Health and Medical Research Institute, Adelaide, Australia.
- 1039 Philipps University and University Hospital of Marburg, Department of Neuropathology, Marburg, Germany.
- 1040 National Institutes of Health, National Institute of Allergy and Infectious Diseases, Laboratory of Immunoregulation, Bethesda, MD, USA.
- 1041 Oregon Health & Science University, Casey Eye Institute, Portland, OR, USA.
- 1042 University of Münster, University Hospital, Department of Neurology with Institute of Translational Neurology, Münster, Germany.
- 1043 University of Illinois at Urbana-Champaign, Department of Molecular and Integrative Physiology, Urbana, IL, USA.
- 1044 Weill Cornell Medicine, Departments of Pediatrics and Cell and Developmental Biology, New York, NY, USA.
- 1045 Metabolomics and Cell Biology Platforms, Gustave Roussy Comprehensive Cancer Institute, Villejuif, France; Equipe 11 labellisée Ligue contre le Cancer, Centre de Recherche des Cordeliers, INSERM U 1138, Paris, France.
- 1046 Queen Mary University of London, Barts Cancer Institute, London, UK.
- 1047 University College London, MRC Laboratory for Molecular Cell Biology, London, UK.
- 1048 Maastricht University Medical Center, GROW School for Oncology, Department of Radiotherapy, Maastricht|, The Netherlands.
- 1049 Ben-Gurion University of the Negev, Faculty of Health Sciences, Department of Clinical Biochemistry and Pharmacology, Beer Sheva, Israel.
- 1050 University of Sadat City, Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, Sadat City, Egypt.
- 1051 Indiana University-Purdue University (IUPUI), Department of Pathology and Laboratory Medicine, Indianapolis, IN, USA.
- 1052 University of Eastern Finland, School of Pharmacy, Kuopio, Finland.
- 1053 Barkatullah University, Department of Biochemistry & Genetics, Bhopal, India.
- 1054 Charité - Universitätsmedizin Berlin, Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Berlin, Germany.
- 1055 Columbia University, Vagelos College of Physicians and Surgeons, Department of Pathology and Cell Biology, New York, NY, USA.
- 1056 Friedrich-Schiller-University Jena, University Hospital Jena, Institute of Human Genetics, Jena, Germany.
- 1057 Gyeongsang National University School of Medicine, Department of Biochemistry and Convergence Medical Sciences and Institude of Health Sciences, Jinju, Republic of Korea.
- 1058 University of Minnesota, Department of Biochemistry, Molecular Biology, and Biophysics, Minneapolis, MN, USA.
- 1059 Kyungpook National University, School of Medicine, Department of Biochemistry and Cell Biology, Cell and Matrix Research Institute, Daegu, Korea.
- 1060 Daegu Gyeongbuk Institute of Science and Technology (DGIST), Department of Brain and Cognitive Sciences, Daegu, Korea.
- 1061 Dankook University, College of Medicine, Department of Pharmacology, Cheonan, Chungnam, Korea.
- 1062 Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cell Factory Research Center, Daejeon, Korea; University of Science and Technology, KRIBB School of Biotechnology, Department of Environmental Biotechnology, Daejeon, Korea.
- 1063 Dankook University, College of Dentistry, Cheonan, Korea.
- 1064 Seoul National University College of Medicine, Department of Biomedical Sciences, and Ophthalmology, Seoul, Korea.
- 1065 Konkuk University, Department of Stem Cell and Regenerative Biotechnology, Seoul, Korea.
- 1066 Kyung Hee University, Department of Oral Biochemistry and Molecular Biology, School of Dentistry, Seoul, Korea.
- 1067 Sookmyung Women's University, Department of Biological Sciences, Seoul, Korea.
- 1068 Korea Research Institute of Chemical Technology, Center for Convergent Research of Emerging Virus Infection, Daejeon, Korea.
- 1069 Penn State College of Medicine, Department of Cellular and Molecular Physiology, Hershey, PA, USA.
- 1070 Weizmann Institute of Science, Department of Molecular Genetics, Rehovot, Israel.
- 1071 New York University School of Medicine, Department of Radiation Oncology, Perlmutter Cancer Center, New York, NY, USA.
- 1072 National Institute of Biomedical Innovation, Health and Nutrition (NIBIOHN), Center for Rare Disease Research, Ibaraki, Osaka, Japan.
- 1073 AstraZeneca, Bioscience, Oncology R&D, Cambridge, UK.
- 1074 University College London, Institute of Healthy Ageing, Department of Genetics, Evolution & Environment, London, UK.
- 1075 University of Utah, Huntsman Cancer Institute, Salt Lake City, UT, USA; University of Utah School of Medicine, Division of Oncology, Department of Internal Medicine, Salt Lake City, UT, USA.
- 1076 Cancer Research UK Cancer Therapeutics Unit, The Institute of Cancer Research London, Sutton, UK.
- 1077 St. Boniface Hospital Albrechtsen Research Centre, Departments of Physiology and Pathophysiology, and Pharmacology and Therapeutics, Max Rady College of Medicine; Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
- 1078 Vavilov Institute of General Genetics RAS, Department of Epigenetics, Moscow, Russia.
- 1079 S&J Kishi Research Corporation, Jupiter, FL, USA.
- 1080 Nihon Pharmaceutical University, Department of Pharmaceutical and Medical Business Sciences, Bunkyoku, Tokyo, Japan.
- 1081 St. Marianna University Graduate School of Medicine, Department of Molecular Neuroscience, Kanagawa, Japan.
- 1082 Nagasaki University, Institute of Biomedical Sciences, Department of Clinical Research Pharmacy, Nagasaki, Japan.
- 1083 Albert Einstein College of Medicine, Departments of Medicine and Cell Biology and Wilf Family Cardiovascular Research Institute, Bronx, NY, USA.
- 1084 University of Copenhagen, Department of Neuroscience, Copenhagen, Denmark.
- 1085 Perelman School of Medicine at the University of Pennsylvania, Department of Medicine (Hematology-Oncology), Philadelphia, PA, USA.
- 1086 Friedrich-Baur-Institute, Department of Neurology, University of Munich, Munich, Germany.
- 1087 University Hospital Erlangen, Department of Molecular Neurology, Erlangen, Germany.
- 1088 University of Oslo, Centre for Cancer Cell Reprogramming, Institute of Clinical Medicine, Faculty of Medicine, Montebello, Norway; Oslo University Hospital, Department of Molecular Cell Biology, Institute for Cancer Research, Montebello, Norway.
- 1089 The University of Tokyo, Department of Biochemistry and Molecular Biology, Graduate School of Medicine, Tokyo, Japan.
- 1090 The Hong Kong Polytechnic University, Department of Applied Biology and Chemical Technology, Hong Kong, China.
- 1091 Icahn School of Medicine at Mount Sinai, Department of Geriatrics and Palliative Medicine, New York, NY, USA.
- 1092 The University of Tokyo, Institute for Quantitative Biosciences, Laboratory of RNA Function, Tokyo, Japan; Albert Einstein College of Medicine, Department of Anatomy and Structural Biology, Bronx, NY, USA.
- 1093 New York Institute of Technology, College of Osteopathic Medicine, Department of Biomedical Sciences, Old Westbury, NY, USA.
- 1094 Johann Wolfgang Goethe-University Frankfurt am Main, Faculty of Computer Science and Mathematics, Institute of Computer Science, Frankfurt am Main, Germany.
- 1095 University Medicine Göttingen, Clinic for Neurology, Göttingen, Germany.
- 1096 University Hospital Münster (UKM), Institute of Musculoskeletal Medicine, Münster, Germany.
- 1097 Goethe University Hospital Frankfurt, Neuroscience Center, Experimental Neurosurgery, Frankfurt/Main, Germany.
- 1098 Division of Brain Disease Research, Korea National Institute of Health, Cheongju-si, Republic of Korea.
- 1099 Juntendo University Graduate School of Medicine, Department of Cell Biology and Neuroscience, Hongo, Bunkyo-ku, Tokyo, Japan.
- 1100 University of Graz, Institute of Molecular Biosciences, BioTechMed-Graz, Graz, Austria.
- 1101 Juntendo University School of Medicine, Department of Physiology, Bunkyo-ku, Tokyo, Japan.
- 1102 Sapporo Higashi Tokushukai Hospital, Advanced Surgery Center, Sapporo, Hokkaido, Japan.
- 1103 The Abigail Wexner Research Institute at Nationwide Children's Hospital, Center for Microbial Pathogenesis, Columbus, OH, USA.
- 1104 Earlham Institute, Norwich, UK.
- 1105 Newcastle University, Ageing Research Laboratories, Institute for Cell and Molecular Biosciences, Newcastle upon Tyne, UK.
- 1106 Norwegian University of Science and Technology (NTNU), Department of Biotechnology and Food Science, Trondheim, Norway; Korsnes Biocomputing (KoBio), Trondheim, Norway.
- 1107 University of Eastern Finland, Department of Ophthalmology, Kuopio, Finland.
- 1108 Indiana University School of Medicine, Indianapolis, IN, USA.
- 1109 Hiroshima University, Graduate School of Biomedical and Health Sciences, Hiroshima, Japan.
- 1110 China National Rice Research Institute, State Key Laboratory of Rice Biology, Hangzhou. China.
- 1111 Democritus University of Thrace, Department of Radiotherapy/Oncology, Alexandroupolis, Greece.
- 1112 Kanazawa Medical University, Department of Diabetology & Endocrinology, Uchinadacho, Ishikawa, Japan.
- 1113 Fukuoka University, Faculty of Pharmaceutical Sciences, Department of Biochemistry, Fukuoka, Japan.
- 1114 University of Freiburg, Institute of Biochemistry and Molecular Biology, ZBMZ, Faculty of Medicine, Freiburg, Germany; University of Freiburg, CIBSS - Centre for Integrative Biological Signalling Studies, Freiburg, Germany.
- 1115 Northwestern University Feinberg School of Medicine, Department of Neurology, Chicago, IL, USA.
- 1116 University of Texas, Southwestern Medical Center, Dallas, TX, USA.
- 1117 Queen's University of Belfast, Wellcome-Wolfson Institute for Experimental Medicine, Belfast, UK.
- 1118 Université Lyon, Université Claude Bernard Lyon 1, CNRS UMR 5310, INSERM U1217, Institut NeuroMyoGène, Lyon, France.
- 1119 Université de Paris, Sorbonne Université, INSERM U1138, Centre de Recherche des Cordeliers, Equipe labellisée par la Ligue contre le cancer, Paris, France; Institut Gustave Roussy, Metabolomics and Cell Biology Platforms, Villejuif, France; Hôpital Européen Georges Pompidou, AP-HP, Pôle de Biologie, Paris, France; Chinese Academy of Medical Sciences, Suzhou Institute for Systems Medicine, Suzhou, China; Karolinska Institute, Karolinska University Hospital, Department of Women's and Children's Health, Stockholm, Sweden.
- 1120 Babraham Institute, Babraham, Cambridge, UK.
- 1121 Tokyo University of Science, Department of Applied Biological Science, Noda, Chiba, Japan.
- 1122 VIB-KU Leuven Center for Brain & Disease Research, Leuven, Belgium; KU Leuven, Department of Neurosciences, Leuven Brain Institute, Leuven, Belgium.
- 1123 University of Bonn, LIMES Institute, Bonn, Germany.
- 1124 Emory University, Department of Pharmacology and Chemical Biology, School of Medicine, Atlanta, GA, USA.
- 1125 PK-PD-Toxicology and Formulation Division, CSIR-Indian Institute of Integrative Medicine, Jammu, India.
- 1126 University of Houston, Department of Pharmacological and Pharmaceutical Sciences, Houston, TX, USA.
- 1127 Julius L. Chambers Biomedical/Biotechnology Research Institute (BBRI) and North Carolina Central University, Department of Pharmaceutical Sciences, Durham, NC, USA.
- 1128 International Centre for Genetic Engineering and Biotechnology, Cellular Immunology Group, Aruna Asaf Ali Marg, New Delhi, India.
- 1129 Shiga University of Medical Science, Department of Medicine, Otsu, Shiga, Japan.
- 1130 Kalinga institute of industrial technology (KIIT-DU), School of Biotechnology, Campus 11, Patia, Bhubaneswar, Odisha, India.
- 1131 St. Jude Children's Research Hospital, Department of Pathology, Memphis, TN, USA.
- 1132 Indian Institute of Technology (IIT) Guwahati, Department of Biosciences and Bioengineering, Guwahati, Assam, India.
- 1133 Virginia Commonwealth University, Department of Computer Science, Richmond, VA, USA.
- 1134 University of Colorado Anschutz Medical Campus, Department of Pharmacology, Aurora, CO, USA.
- 1135 Sabanci University Nanotechnology Research and Application Center (SUNUM), Tuzla, Istanbul, Turkey.
- 1136 Wonkwang University School of Medicine, Zoonosis Research Center, Iksan, Jeonbuk, Korea.
- 1137 Keimyung University, School of Medicine, Department of Immunology, Daegu, Korea.
- 1138 Protein Metabolism Medical Research Center and Department of Biomedical Sciences, Seoul National University School of Medicine, Seoul, Korea.
- 1139 University of California, Irvine School of Medicine, Irvine, CA, USA.
- 1140 INRS, INRS-Institut Armand-Frappier. Montréal, QC, Canada.
- 1141 University of Burgundy, Centre Georges François Leclerc, Department of Medical Oncology, Dijon, France.
- 1142 University of Ferrara, Department of Translational Medicine, Ferrara, Italy.
- 1143 Cellular Microbiology and Physics of Infection Group, Center for Infection and Immunity of Lille, Institut Pasteur de Lille, CNRS, INSERM, CHU Lille, University of Lille, Lille Cedex, France.
- 1144 University of Ottawa, Department of Cellular & Molecular Medicine, Ottawa, Ontario, Canada.
- 1145 Huazhong Agricultural University, National Key Laboratory of Crop Genetic Improvement, Wuhan, China.
- 1146 Macquarie University, Faculty of Medicine, Health and Human Sciences, Department of Biomedical Science, Centre for Motor Neuron Disease Research, Sydney, NSW, Australia.
- 1147 University of California, San Francisco Department of Ophthalmology, San Francisco, CA, USA.
- 1148 National Yang-Ming University, Department of Life Sciences and Institute of Genome Sciences, Taipei, Taiwan.
- 1149 Yale University, Department of Cellular and Molecular Physiology, New Haven, CT, USA.
- 1150 School of Biochemistry, University of Bristol, Bristol, UK.
- 1151 Penn State College Medicine, Department of Cellular and Molecular Physiology, Hershey, PA, USA.
- 1152 University of Greifswald, Institute of Pharmacy, Greifswald, Germany.
- 1153 Stockholm University, Department of Biochemistry and Biophysics, Stockholm, Sweden.
- 1154 Johannes Kepler University Linz, Kepler University Hospital GmbH, Institute of Pathology and Molecular Pathology, Linz, Austria.
- 1155 University Bourgogne Franche-Comté, AgroSup Dijon, PAM UMR A 02.102, Dijon, France.
- 1156 IGBMC, Inserm U1258, Cnrs UMR7104, Strasbourg University, Illkirch, France.
- 1157 Mayo Clinic, Department of Medicine, Division of Gastroenterology and Hepatology, Rochester, MN, USA.
- 1158 University of Virginia, Department of Cell Biology, Charlottesville, VA, USA.
- 1159 Macau University of Science and Technology, State Key Laboratory of Quality Research in Chinese Medicine, Taipa, Macau, China.
- 1160 The Hong Kong Polytechnic University, Faculty of Health and Social Sciences, Department of Health Technology and Informatics, Hunghom, Hong Kong.
- 1161 University of Nottingham, School of Life Sciences, Nottingham, UK.
- 1162 Sichuan Academy of Medical Science & Provincial Hospital, Medical School of UESTC, Chengdu, China.
- 1163 Université Paris Saclay, Institut des Neurosciences Paris-Saclay (Neuro-PSI)-CNRS UMR 9197, Orsay France.
- 1164 McGill University Health Center, Department of Medicine, Cancer Research Program, Montreal, QC, Canada.
- 1165 Research Institute of the McGill University Health Centre, Meakins-Christie Laboratories and Translational Research in Respiratory Diseases Program, Montréal, Québec, Canada; McGill University, Department of Critical Care and Division of Experimental Medicine, Montréal, Québec, Canada; UQAM, Faculté des Sciences, Département des Sciences de l'activité physique, Montréal, Canada.
- 1166 Ulsan National Institute of Science and Technology, Department of Biological Sciences, Ulsan, Korea.
- 1167 National Taipei University of Nursing and Health Sciences, School of Nursing, Taipei, Taiwan.
- 1168 KAIST, Department of Biological Sciences, Daejon, Korea.
- 1169 Konkuk University, School of Medicine, Department of Anatomy, Seoul, Korea.
- 1170 Korea Advanced Institute of Science and Technology, Graduate School of Medical Science and Engineering, Daejeon, Korea.
- 1171 QIMR Berghofer Medical Research Institute, Herston, Queensland, Australia.
- 1172 Hannam University, Department of Biological Sciences and Biotechnology, Daejeon, South Korea.
- 1173 Chungnam National University, Graduate School of Analytical Science and Technology (GRAST), Daejeon 305-764, Republic of Korea.
- 1174 University of Michigan, Department of Molecular & Integrative Physiology, Ann Arbor, MI, USA.
- 1175 Incheon National University, College of Life Sciences and Bioengineering, Division of Life Sciences, Incheon, Korea.
- 1176 Yonsei University College of Medicine, Department of Pharmacology, Seoul, Korea.
- 1177 Seoul National University College of Medicine, Department of Biochemistry and Molecular Biology, Seoul, Korea.
- 1178 Yonsei University College of Medicine, Severance Biomedical Science Institute and Department of Internal Medicine, Seoul, Korea.
- 1179 Ajou University Graduate School of Medicine, Department of Biomedical Sciences, Suwon, Gyeonggi, Korea.
- 1180 Seoul National University College of Medicine, Department of Biomedical Sciences, Neuroscience Research Institute, Seoul, Korea.
- 1181 University of Kansas Medical Center, Department of Biochemistry and Molecular Biology, Kansas City, KS, USA.
- 1182 DGIST, Department of Brain & Cognitive Sciences, Daegu, Korea.
- 1183 University of Arizona College of Medicine, Department of Basic Medical Sciences, Phoenix, AZ, USA.
- 1184 Ditmanson Medical Foundation Chia-Yi Christian Hospital, Translational Medicine Research Center, Chiayi City, Taiwan.
- 1185 Yonsei University College of Medicine, Department of Internal Medicine, Seoul, Korea.
- 1186 University of West Florida, Department of Movement Sciences and Health, Pensacola, FL, USA.
- 1187 University of Michigan, Rogel Cancer Center, Department of Periodontics and Oral Medicine, Department of Otolaryngology - Head and Neck Surgery, Ann Arbor, MI, USA.
- 1188 National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
- 1189 University of Maribor, Faculty of Medicine, Maribor, Slovenia; University of Maribor, Faculty of Natural Sciences and Mathematics, Department of Biology, Maribor, Slovenia; University of Maribor, Faculty of Chemistry and Chemical Engineering, Maribor, Slovenia; Medical University of Graz, Division of Cell Biology, Histology and Embryology, Gottfried Schatz Research Center, Graz, Austria.
- 1190 Medical University of Graz, Division of Cell Biology, Histology and Embryology, Gottfried Schatz Research Center, Graz, Austria.
- 1191 Guangzhou Municipal and Guangdong Provincial Key Laboratory of Protein Modification and Degradation, School of Basic Medical Sciences, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, China.
- 1192 Institut National de la Santé et de la Recherche Médicale (Inserm), Université de Paris, Paris Cardiovascular Center - PARCC, Paris, France.
- 1193 Universidade Federal do Rio Grande do Sul (UFRGS), Center of Biotechnology and Department of Biophysics, Porto Alegre, RS, Brazil.
- 1194 USC Norris Comprehensive Cancer Center , Los Angeles, CA, USA.
- 1195 University of Pisa, Department of Translational Research and New Technologies in Medicine and Surgery, Pisa, Italy.
- 1196 Universidad Autónoma de Madrid, Department of Biology, Madrid, Spain.
- 1197 University of São Paulo, School of Pharmaceutical Sciences of Ribeirão Preto, Department of Clinical Analyses, Toxicology and Food Sciences, Ribeirão Preto, SP, Brazil.
- 1198 University of Luxembourg, Department of Life Sciences and Medicine, Molecular Disease Mechanisms Group, Belval, Luxembourg.
- 1199 The Chinese University of Hong Kong, Faculty of Medicine, School of Biomedical Sciences, Shatin, New Territories, Hong Kong.
- 1200 Icahn School of Medicine at Mount Sinai, Department of Medicine, New York, NY, USA.
- 1201 Royal Veterinary College, London, UK; UCL Queen Square Institute of Neurology, Department of Neurodegenerative Disease, London, UK.
- 1202 La Jolla Institute for Immunology and University of California San Diego, Laboratory of Inflammation Biology, La Jolla, CA, USA.
- 1203 College of Life Science and Technology, Jinan University, Guangzhou, China.
- 1204 Fudan University, Shanghai Cancer Center and Institutes of Biomedical Sciences, Shanghai, China.
- 1205 Sun Yat-sen University, Sun Yat-sen Memorial Hospital, Department of Pathology, Guangzhou, Guangdong Province, China.
- 1206 Sichuan University, West China Hospital, State key laboratory of biotherapy, Chengdu, Sichuan, China.
- 1207 Virginia Polytechnic Institute, Department of Biological Sciences, Blacksburg, VA, USA.
- 1208 Children's Hospital of Soochow University, Institute of Pediatric Research, Suzhou, China.
- 1209 Department of Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, MI, USA.
- 1210 Jinan University, College of Life Science and Technology, Department of Biology, Guangzhou, China.
- 1211 Virginia Commonwealth University, Departement of Pharmacology and Toxicology, Richmond, VA, USA.
- 1212 Fudan University, Hospital of Obstetrics and Gynecology, Institute of Obstetrics and Gynecology, Laboratory for Reproductive Immunology, Shanghai, China.
- 1213 South China Normal University, School of Life Sciences, Institute of Insect Science and Technology, Guangdong Provincial Key Laboratory of Insect Developmental Biology and Applied Technology, Guangzhou, China.
- 1214 Emory University School of Medicine, Department of Pharmacology and Chemical Biology, Atlanta, GA, USA.
- 1215 Boston Children's Hospital, Harvard Medical School, Departments of Urology and Surgery, Boston, MA, USA.
- 1216 Stanford University, Department of Gastroenterology & Hepatology, Stanford, CA, USA.
- 1217 Chinese Academy of Agricultural Sciences, Institute of Plant Protection, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Beijing, China.
- 1218 Chinese Academy of Agriculture Sciences, Lanzhou Veterinary Research Institute, State Key Laboratory of Veterinary Etiological Biology, Lanzhou, Gansu, China.
- 1219 Chinese Academy of Sciences, Hefei Institutes of Physical Science, High Magnetic Field Laboratory, Hefei, Anhui Province, China.
- 1220 Army Medical University, Department of Bichemistry and Molecular Biology, Chongqing, Chongqing, China.
- 1221 The Wistar Institute, Molecular and Cellular Oncogenesis Program, Philadelphia, PA, USA.
- 1222 Sun Yat-sen University, The 3rd Affiliated Hospital of Sun Yat-sen University, Vaccine Research Institute, Guangzhou, China.
- 1223 Nanjing Agricultural University, College of Life Sciences, Key Laboratory of Agricultural Environmental Microbiology of Ministry of Agriculture and Rural Affairs, Nanjing , China.
- 1224 Free University of Berlin, Department of Biology, Chemistry and Pharmacy, Berlin, Germany.
- 1225 Northwest A&F University, College of Life Sciences, Yangling, Shaanxi, China.
- 1226 Academia Sinica, Institute of Cellular and Organismic Biology, Taipei, Taiwan.
- 1227 Nathan S. Kline Institute, Center for Dementia Research, Orangeburg, NY, USA.
- 1228 National Institute of Child Health and Human Development, National Institutes of Health, Cell Biology and Neurobiology Branch, Bethesda, MD, USA.
- 1229 Konkuk University, Department of Veterinary Medicine, Seoul, Korea.
- 1230 São Paulo State University, Botucatu Medical School, Center for Evaluation of Environmental Impact on Human Health (TOXICAM), Department of Pathology, Botucatu, SP, Brazil.
- 1231 San Raffaele Open University and IRCCS San Raffaele Pisana, Laboratorio di Patologia Cellulare e Molecolare, Rome, Italy.
- 1232 First Affiliated Hospital of Nanjing Medical University, Department of Neurosurgery, Nanjing, China; Jiangsu Province Hospital, Department of Neurosurgery, Nanjing, China.
- 1233 Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-Da Hospital, Kaohsiung, Taiwan; I-Shou University, College of Medicine, School of Medicine, Kaohsiung, Taiwan.
- 1234 Mackay Memorial Hospital, Department of Pediatrics, Taipei, Taiwan; Mackay Medical College, Department of Medicine, New Taipei,Taiwan.
- 1235 Zhejiang Academy of Agricultural Sciences, Institute of Plant Protection and Microbiology, State Key Laboratory for Managing Biotic and Chemical Treatments to the Quality and Safety of Agro-products, Hangzhou, China; Zhejiang University, Institute of Biotechnology, Hangzhou, China.
- 1236 Life Sciences Institute and Department of Cell & Developmental Biology, University of Michigan, Ann Arbor, MI, USA.
- 1237 National Health Research Institutes, Institute of Biomedical Engineering and Nanomedicine, Zhunan, Miaoli, Taiwan.
- 1238 Chang Gung Memorial Hospital, Liver Research Center, Linkou, Taoyuan, Taiwan; Chang Gung University, College of Medicine, Graduate Institute of Biomedical Sciences, Taoyuan, Taiwan; Chang Gung University of Science and Technology, College of Human Ecology, Research Center for Chinese Herbal Medicine, Taoyuan, Taiwan.
- 1239 The Ohio State University, Wexner Medical Center, Department of Surgery, Columbus, OH, USA.
- 1240 School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, China.
- 1241 Huazhong University of Science and Technology, College of Life Science and Technology, Hubei Bioinformatics and Molecular Imaging Key Laboratory, Key Laboratory of Molecular Biophysics of Ministry of Education, Wuhan, Hubei, China.
- 1242 Harvard Medical School, Massachusetts General Hospital, Gastrointestinal Unit/Medicine, Boston, MA, USA.
- 1243 Mayo Clinic, Department of Biochemistry and Molecular Biology, Rochester, MN, USA.
- 1244 Brigham and Women's Hospital, Harvard Medical School, Center for Nanomedicine and Department of Anesthesiology, Boston, MA, USA.
- 1245 Instituto de Biofisica da UFRJ, Rio de Janeiro, Brazil.
- 1246 Technical University of Munich, School of Medicine, Klinikum rechts der Isar, Department of Neurology, Munich, Germany.
- 1247 Indiana University School of Medicine, Department of Pediatrics and Center for Diabetes and Metabolic Diseases, Indianapolis, IN, USA.
- 1248 University of Singapore, Department of Biological Sciences, Singapore.
- 1249 University of Maryland School of Medicine, Department of Anesthesiology, Baltimore, MD, USA.
- 1250 University of Iowa, Fraternal Order of Eagles Diabetes Research Center, Obesity Research and Education Initiative, Abboud Cardiovascular Research Center, Department of Health and Human Physiology, Iowa City, IA, USA.
- 1251 Poznan University of Medical Sciences, Department of Clinical Chemistry and Molecular Diagnostics, Poznan, Poland.
- 1252 Duke University, Department of Ophthalmology, Durham, NC, USA.
- 1253 Department of Microbiology & Immunology, Dalhousie University, Halifax, Nova Scotia, Canada.
- 1254 Institute of Neuroscience of Soochow University, Suzhou, China.
- 1255 Chinese Academy of Sciences, Center for Biosafety Mega-Science, Institute of Microbiology, CAS Key Laboratory of Pathogenic Microbiology and Immunology, Chaoyang District, Beijing, China.
- 1256 Nankai University, Department of Microbiology, Tianjin, China.
- 1257 Guangdong Medical University, Institute of Nephrology, and Zhanjiang Key Laboratory of Prevention and Management of Chronic Kidney Disease, Zhanjiang, Guangdong, China.
- 1258 Capital Medical University, Beijing Institute for Brain Disorders, Beijing, China.
- 1259 Central South University, School of Life Sciences, Molecular Biology Research Center, Changsha, Hunan, China.
- 1260 The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- 1261 Columbia University, Naomi Berrie Diabetes Center, Department of Pathology and Cell Biology, College of Physicians and Surgeons, New York, NY, USA.
- 1262 University of New Mexico Health Sciences Center, Department of Biochemistry and Molecular Biology, Albuquerque, NM, USA.
- 1263 Henan Academy of Agricultural Sciences, Institute of Plant Nutrition, Agricultural Resources and Environmental Science, Jinshui District, Zhengzhou, Henan, China.
- 1264 Zhejiang University School of Medicine, Department of Biochemistry and Department of Cardiology of the Second Affiliated Hospital, Hangzhou, Zhejiang, China.
- 1265 Zhejiang University, Biotechnology Institute, State Key Laboratory for Rice Biology, Hangzhou, China.
- 1266 CAS Key Laboratory of Regenerative Biology, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China.
- 1267 Harvard Medical School, Department of Microbiology; Brigham and Women's Hospital, Division of Infectious Diseases, Boston, MA, USA.
- 1268 University of Colorado at Boulder, Department of Biochemistry, Boulder, CO, USA.
- 1269 ShanghaiTech University, School of Life Science and Technology, Shanghai, China.
- 1270 Saarland University, Department of Neurology, Homburg, Germany.
- 1271 Johns Hopkins University, Department of Biochemistry and Molecular Biology, Baltimore, MD, USA.
- 1272 Shenyang Pharmaceutical University, Department of Pharmacology, Shenyang, China.
- 1273 Tsinghua Unversity, School of Life Sciences, Beijing, China.
- 1274 University of Texas MD Anderson Cancer Center, Department of Sarcoma Medical Oncology, Houston, TX, USA.
- 1275 University of Bourgogne Franche-Comté, Team 'Biochemistry of the Peroxisome, Inflammation and Lipid Metabolism (EA7270)/Inserm, Faculty of Sciences Gabriel, Dijon, France.
- 1276 Universitat Autonoma de Barcelona, Departament of Biochemistry and Institut Neurosciences, Faculty of Medicine, Bellaterra, Barcelona, Spain.
- 1277 Vall d´Hebron Research Institute, Barcelona, Spain.
- 1278 Oslo University Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Oslo, Norway.
- 1279 Harvard Medical School, Brigham and Women's Hospital, Department of Neurology, Boston, MA, USA.
- 1280 London School of Hygiene & Tropical Medicine, Department of Infection Biology, London, UK.
- 1281 Universidad Autonoma de Madrid, School of Medicine, Department of Pharmacology, Madrid, Spain.
- 1282 Universidad Autónoma de Madrid, Departamento de Biología Molecular, Madrid, Spain and Centro de Biología Molecular Severo Ochoa (CSIC-UAM), Madrid, Spain.
- 1283 Universidad de Zaragoza, IA2, IIS, Laboratorio de Genética Bioquímica (LAGENBIO), Zaragoza, Spain; Universidad de Zaragoza, IA2, IIS, Centro de Encefalopatías y Enfermedades Transmisibles Emergentes, Zaragoza, Spain.
- 1284 University Medical Center Utrecht, Center for Molecular Medicine, Utrecht, The Netherlands; Regenerative Medicine Center, Utrecht, The Netherlands.
- 1285 University of Turin, Department of Veterinary Sciences, Grugliasco (TO), Italy.
- 1286 Université de Paris, Centre de Recherche sur l'Inflammation (CRI), Inserm-UMR1149, Paris, France.
- 1287 Newcastle University, Institute of Translation and Clinical Studies, Precision Medicine, The Medical School, Newcastle upon Tyne, UK.
- 1288 State University of New York at Buffalo, Buffalo, NY, USA.
- 1289 Universidad Mayor, Center for Integrative Biology and Geroscience for Brain, Health and Metabolism, Santiago, Chile.
- 1290 National University of Singapore, Yong Loo Lin School of Medicine, Department of Physiology, Singapore.
- 1291 Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
- 1292 University of Macau, Institute of Chinese Medical Sciences, State Key Laboratory of Quality Research in Chinese Medicine, Taipa, Macao, China.
- 1293 Institute of Virology, University Hospital Essen, Essen, Germany.
- 1294 Pfizer Inc, DSRD, La Jolla, CA, USA.
- 1295 University of St Andrews, School of Medicine, North Haugh, St Andrews, UK.
- 1296 Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal and ICVS/3B's - PT Government Associate Laboratory, Braga/Guimarães, Portugal.
- 1297 Duke University, School of Medicine, Duke Center for Virology, Department of Molecular Genetics and Microbiology, Durham, NC, USA.
- 1298 BC Cancer, Trev and Joyce Deeley Research Centre, Victoria, BC, Canada; University of Victoria, Department of Biochemistry and Microbiology, Victoria, BC, Canada.
- 1299 University of Würzburg, Institute of Clinical Neurobiology, Würzburg, Germany.
- 1300 University of British Columbia, Centre for Heart Lung Innovation/Department of Pathology and Laboratory Medicine, Vancouver, BC, Canada.
- 1301 Kunming Institute of Zoology, Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences & Yunnan Province, Kunming, Yunnan, China.
- 1302 Plymouth University, Peninsula School of Medicine and Dentistry, Plymouth, UK.
- 1303 Huazhong Agricultural University, Laboratory of Molecular Nutrition for Aquatic Economic Animals, Fishery College, Wuhan, China.
- 1304 RWTH Aachen University, Medical School, Institute of Biochemistry and Molecular Biology, Aachen, Germany.
- 1305 International Clinical Research Center, St'Anne University Hospital, Brno, Czech Republic; Institute of Molecular Biology and Biophysics, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Siberia, Russia.
- 1306 University of Oslo, Faculty of Medicine, Institute of Basic Medical Sciences, Department of Molecular Medicine, Oslo, Norway; University of Oslo, Faculty of Medicine, Institute of Clinical Medicine, Centre for Cancer Cell Reprogramming, Oslo, Norway.
- 1307 Justus Liebig University, University Hospital Giessen and Marburg GmbH, Departement of Ophthalmology, Giessen, Germany.
- 1308 The Hong Kong Polytechnic University, Department of Health Technology and Informatics, Hong Kong, China.
- 1309 Shandong Normal University, Shandong Provincial Key Laboratory of Plant Stress, College of Life Sciences, Jinan, Shandong, China.
- 1310 Yale School of Medicine, Department of Cell Biology, New Haven, CT, USA.
- 1311 Guangzhou Medical University, Department of Histology and Embryology, Guangzhou, China.
- 1312 Soochow University, Institute of Neuroscience, Jiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases, Suzhou, Jiangsu, China.
- 1313 Department of Emergency Medicine, Thomas Jefferson University, Philadelphia, PA, USA.
- 1314 Air Force Medical University, Xijing Hospital, Department of Clinical Laboratory, Xi'an, China.
- 1315 Tianjin Medical University, Department of Biochemistry and Molecular Biology; School of Basic Medical Sciences, Tianjin Key Laboratory of Medical Epigenetics, Tianjin, China.
- 1316 Albert Einstein College of Medicine, Department of Pathology. Bronx, NY, USA.
- 1317 Iowa State University, Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology, Ames, IA, USA.
- 1318 Michigan State University, College of Human Medicine, Grand Rapids, MI, USA.
- 1319 Ben May Department for Cancer Research, University of Chicago, Chicago IL, USA.
- 1320 Perelman School of Medicine at the University of Pennsylvania, Department of Neuroscience, Philadelphia, PA, USA.
- 1321 University of Graz, Institute of Molecular Biosciences, BioTechMed-Graz, BioHealth, Graz, Austria.
- 1322 University of Texas Health San Antonio, Department of Medicine, San Antonio, TX, USA.
- 1323 Albert Einstein College of Medicine, Institute for Aging Studies, Department of Developmental and Molecular Biology, Bronx, NY, USA.
- 1324 RIKEN, Laboratory for Retinal Regeneration, Kobe, Hyogo, Japan.
- 1325 Tokyo Medical and Dental University, Department of Cardiovascular Medicine, Tokyo, Japan.
- 1326 Justus-Liebig University, Department of Internal Medicine, Universities of Giessen & Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany.
- 1327 Part overlaps with Costanza Montagna: Danish Cancer Society Research Center, Computational Biology Laboratory, Copenhagen, Denmark; Danish Cancer Society Research Center, Computational Biology Laboratory, Copenhagen, Denmark.
- 1328 Cellular and Molecular Signaling, New York, NY, USA.
- 1329 Central Michigan University, Department of Psychology and Neuroscience Program, Mt. Pleasant, MI, USA.
- 1330 Inserm U1138, Centre de Recherche des Cordeliers, Sorbonne Université, Université de Paris, Paris, France.
- 1331 University of Naples Federico II, Department of Biology, Naples, Italy.
- 1332 Washington University in St. Louis, Department of Cell Biology and Physiology, St. Louis, MO, USA.
- 1333 CSIR - Indian Institute of Integrative Medicine, Department of Cancer Pharmacology, Sanat Nagar, Srinagar, Jammu and Kashmir, India.
- 1334 National University of Singapore, Yong Loo Lin School of Medicine, Department of Physiology, Mitochondrial Physiology and Metabolism Lab, Singapore; National University of Singapore, NUHS Centre for Healthy Ageing, Singapore.
- 1335 Center for Global Health, Catholic University of the Sacred Heart, Rome, Italy.
- 1336 Oregon Health and Science University, Knight Cardiovascular Institute, Portland, OR, USA.
- 1337 Iowa State University, Department of Biomedical Sciences, Ames, IA, USA.
- 1338 The Chinese University of Hong Kong, The Prince of Wales Hospital, The Faculty of Medicine, Department of Orthopaedics and Traumatology, New Territories, Shatin, Hong Kong, China.
- 1339 Dana-Farber Cancer Institute, Division of Genomic Stability and DNA Repair, Department of Radiation Oncology, Boston, MA, USA.
- 1340 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany; CAESAR Research Center, Bonn, Germany.
- 1341 Vita-Salute San Raffaele University & IRCCS San Raffaele Scientific Institute, Division of Immunology, Transplantation and Infectious Diseases, Milano, Italy.
- 1342 Virginia Commonwealth University School of Medicine, VCU Institute of Molecular Medicine, Massey Cancer Center, Department of Microbiology and Immunology, Richmond, VA, USA.
- 1343 Molecular Biology and Genetics Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India.
- 1344 KU Leuven, Department of Imaging and Pathology, Leuven, Belgium.
- 1345 University College London, School of Pharmacy, Department of Pharmacology, London, United Kingdom.
- 1346 Massachusetts General Hospital, Department of Molecular Biology, Boston, MA, USA.
- 1347 Université Côte d'Azur, INSERM, C3M, Nice, France.
- 1348 Presidio San Paolo Polo Universitario, Dipartimento di Scienze della Salute San Paolo, Milano, Italy.
- 1349 University of Milan, Department of Pharmacological and Biomolecular Sciences, Milan, Italy.
- 1350 Consiglio Nazionale delle Ricerche, Istituto di Biochimica e Biologia Cellulare, Monterotondo scalo (RM), Italy.
- 1351 University of Camerino, School of Pharmacy, Camerino, MC, Italy.
- 1352 University of Oviedo, Principality of Asturias Institute for Biomedical Research, Department of Functional Biology, Oviedo, Spain.
- 1353 Washington University in St. Louis, Department of Biology, St. Louis, MO, USA.
- 1354 UMBC, Department of Chemical, Biochemical and Environmental Engineering, Baltimore, MD, USA.
- 1355 University of Vienna, Max Perutz Labs, Department of Biochemistry and Cell Biology, Vienna BioCenter, Vienna, Austria.
- 1356 Harvard Medical School, Department of Biological Chemistry and Molecular Pharmacology, Boston, MA, USA.
- 1357 European Institute of Oncology (IEO) IRCCS, Department of Experimental Oncology, Milan, Italy; University of Milano, Department of Oncology and Hemato-Oncology, Milan, Italy.
- 1358 KU Leuven, Department of cellular and Molecular Medicine, Laboratory of cellular transport systems, Leuven, Belgium.
- 1359 Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), Department of Biotechnology, Madrid, Spain.
- 1360 Laboratorio de Genética Bioquímica (LAGENBIO), Centro de Encefalopatías y Enfermedades Transmisibles Emergentes, Instituto Agroalimentario de Aragón-IA2, Instituto de Investigación Sanitaria Aragón-IISA, Universidad de Zaragoza, Zaragoza, Spain; Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Instituto Carlos III, Zaragoza, Spain.
- 1361 University of Las Palmas de Gran Canaria, Department of Physical Education and Research Institute of Biomedical and Health Sciences (IUIBS), Las Palmas de Gran Canaria, Spain.
- 1362 Instituto de Investigaciones Biomédicas "Alberto Sols", CSIC-UAM, Madrid, Spain.
- 1363 Cell and Developmental Biology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
- 1364 University of Cambridge, MRC Mitochondrial Biology Unit, Cambridge, UK.
- 1365 National Institutes of Health, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA.
- 1366 Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, AC, Unidad de Biotecnología Médica y Farmacéutica, Guadalajara, Jalisco, México.
- 1367 Albert Einstein College of Medicine, Radiation Oncology, Bronx, NY, USA.
- 1368 Vall d'Hebron research Institute-UAB/Neurodegenerative Diseases Group, Barcelona, Spain.
- 1369 University of São Paulo, Institute of Biomedical Sciences, Department of Anatomy, Laboratory of Functional Neuroanatomy of Pain, São Paulo, Brazil.
- 1370 Laboratory of Immunoendocrinology, Department of Clinical and Toxicological Analyses, University of São Paulo, São Paulo, Brazil.
- 1371 Universidade Anhanguera de São Paulo, Programa de Pós-graduação Stricto sensu e Pesquisa, São Paulo, Brazil.
- 1372 Università Cattolica del Sacro Cuore, Rome, Italy.
- 1373 Institut Jean-Pierre Bourgin, INRAE, AgroParisTech, Université Paris-Saclay, 78000 Versailles, France.
- 1374 University of Minnesota, Department of Biochemistry, Molecular Biology and Biophysics and Department of Medicine, Minneapolis, MN, USA.
- 1375 Università degli Studi di Milano, Dipartimento di Scienze della Salute, Milano, Italy.
- 1376 Universidad Nacional Autónoma de México, Instituto de Fisiología Celular, División de Neurociencias, Departamento de Neuropatología Molecular, Mexico, DF, Mexico.
- 1377 MRC Laboratory of Molecular Biology, Cambridge, UK.
- 1378 University of Rome "Sapienza", Department of Experimental Medicine, Rome, Italy.
- 1379 Center for gender-specific medicine, Italian National Health Institute, Rome, Italy.
- 1380 Biodonostia Health Research Institute, group of Cellular Oncology; IKERBASQUE; CIBERfes, Spain.
- 1381 Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Department of Cardiovascular Medicine, Kyoto, Japan.
- 1382 CHU Clermont-Ferrand, Chirurgie Gynécologique, Clermont-Ferrand, France; Université Clermont Auvergne, Institut Pascal, UMR6602, CNRS/UCA/SIGMA, Clermont-Ferrand, France.
- 1383 University of Chieti-Pescara, Department of Medical, Oral, and Biotechnological Science, Chieti, Italy.
- 1384 University of California, Davis, Department of Dermatology, Sacramento, CA, USA.
- 1385 CONACYT - Centro de Investigación Biomédica de Oriente, Instituto Mexicano del Seguro Social, Puebla, Mexico.
- 1386 IRCCS "Casa Sollievo della Sofferenza", Opera di Padre Pio da Pietrelcina, Division of Internal Medicine and Chronobiology Unit, Department of Medical Sciences, San Giovanni Rotondo (FG), Italy.
- 1387 Sapienza University of Rome, Department of Biology and Biotechnology C. Darwin, Rome, Italy.
- 1388 University of Texas Health Science Center at Houston, Center for Stem Cell and Regenerative Disease, Institute of Molecular Medicine (IMM), Houston, TX, USA.
- 1389 Cleveland Clinic, Lerner Research Institute, Department of Inflammation & Immunity, Cleveland, OH, USA.
- 1390 University of Arkansas for Medical Sciences, Fay W. Boozman College of Public Health, Department of Environmental and Occupational Health, Little Rock, AR, USA.
- 1391 University College Cork, Department of Cancer Research, Cork, Ireland.
- 1392 Academic Decency, MeTooSTEM, Nashville, TN, USA.
- 1393 Mayo Clinic, Rochester, MN, USA.
- 1394 Translational Stem Cell Biology Metabolism Program, Research Programs Unit, University of Helsinki, Helsinki, Finland.
- 1395 Department of Anatomy, Faculty of Medicine, Helsinki, Finland.
- 1396 PSL Research University, Equipe labelisée par la Ligue Contre le Cancer, Institut Curie, Inserm, U830, Stress and Cancer laboratory, Paris, France.
- 1397 Sprott Centre for Stem Cell Research, Ottawa Hospital Research Institute, and University of Ottawa, Department of Medicine, Ottawa, ON, Canada.
- 1398 University of Szeged, Department of Medical Microbiology and Immunobiology, Szeged, Hungary.
- 1399 University of Arkansas for Medical Sciences and Central Arkansas Veterans Healthcare System, Little Rock, AR, USA.
- 1400 University of Amsterdam, Medical Biochemistry, Amsterdam UMC, Amsterdam, The Netherlands.
- 1401 Leiden University, Institute of Biology Leiden, Leiden, The Netherlands; The City University of New York, Queens College, The Graduate Center of the City University of New York, Department of Biology, Flushing, NY, USA.
- 1402 UiT The Arctic University of Norway, Department of Pharmacy, Pharmacology Research Group, Tromso, Norway.
- 1403 Childrens' Hospital, Hannover Medical School, Hannover, Germany.
- 1404 Anhui Province Key Laboratory of Medical Physics and Technology, Center of Medical Physics and Technology, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei, Anhui, China; University of Science and Technology of China, Anhui, China.
- 1405 Laboratory of Cell Biology, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
- 1406 Indian Institute of Technology Delhi, Kusuma School of Biological Sciences, New Delhi, India.
- 1407 University of Cincinnati, Division of Hematology/Oncology, Cincinnati, OH, USA.
- 1408 University of Pretoria, School of Medicine, Faculty of Health Sciences, Department of Physiology, Pretoria, South Africa.
- 1409 Laboratoire d'Optique et Biosciences, Ecole Polytechnique, CNRS, Inserm, Institut Polytechnique de Paris, Palaiseau, France.
- 1410 University of New Mexico, Clinical and Translational Sciences Center, Albuquerque, NM, USA.
- 1411 Carité-Universitaetsmedizin Berlin, Campus Mitte, Institut für Integrative Neuroanatomie, Berlin, Germany.
- 1412 Ruprecht-Karls University of Heidelberg, Women's Hospital, Department of Gynecological Endocrinology and Fertility Disorders, Heidelberg, Germany.
- 1413 Institut Jean-Pierre Bourgin, INRA, AgroParisTech, CNRS, Université Paris-Saclay, Versailles, France.
- 1414 University of Duisburg-Essen, Centre for Medical Biotechnology, Faculty of Biology, Essen, Germany.
- 1415 Tianjin Medical University, Department of Immunology, School of Basic Medical Sciences, Tianjin, China.
- 1416 INSERM Institute of Metabolic and Cardiovascular Diseases (I2MC), Université de Toulouse, Toulouse, France.
- 1417 Shandong University, School of Life Science, Shandong Provincial Key Laboratory of Animal Cells and Developmental Biology, Qingdao, China.
- 1418 Fondazione IRCCS Casa Sollievo della Sofferenza, Poliambulatorio Giovanni Paolo II, Division of Medical Genetics, San Giovanni Rotondo (FG), Italy.
- 1419 Hirosaki University Graduate School of Medicine, Institute of Brain Science, Department of Neuropathology, Hirosaki, Japan; UCL Queen Square Institute of Neurology, Queen Square Brain Bank for Neurological Disorders, London, UK.
- 1420 National Medicines Institute, Department of Drug Biotechnology and Bioinformatics, Warszawa, Poland.
- 1421 University of Washington School of Medicine, Department of Biochemistry, Seattle, WA, USA.
- 1422 University of Washington, Departments of Medicine, Microbiology and Genome Sciences, Seattle, WA, USA.
- 1423 Sage Therapeutics, Cambridge, MA, USA.
- 1424 European Neuroscience Institute (ENI) - A Joint Initiative of the University Medical Center Göttingen and the Max Planck Society, Göttingen, Germany.
- 1425 Swedish University of Agricultural Sciences and Linnean Center for Plant Biology, Department of Molecular Sciences, Uppsala BioCenter, Uppsala, Sweden; Heidelberg University, Center for Organismal Studies, Neuenheimer Feld, Heidelberg, Germany.
- 1426 Kashan University of Medical Sciences, Research Center for Biochemistry and Nutrition in Metabolic Disease, Kashan, Iran.
- 1427 Tehran University of Medical Sciences, School of Medicine, Department of Medical Immunology, Tehran, Iran.
- 1428 Macquarie University, Faculty of Medicine and Health Sciences, New South Wales, Australia.
- 1429 Indian Institute of Technology Jodhpur, Cellular and Molecular Neurobiology Unit, Jodhpur, Rajasthan, India.
- 1430 Amrita Vishwa Vidyapeetham, School of Biotechnology, Kollam, Kerala, India.
- 1431 University of Nebraska Medical Center, Department of Cellular and Integrative Physiology, Omaha, NE, USA.
- 1432 University of Campania L. Vanvitelli, Department of Precision Medicine, Naples, Italy; Institute of Genetic Research, Biogem scarl, Laboratory of Molecular and Precision Oncology, Ariano Irpino (AV), Italy.
- 1433 University of Campania "Luigi Vanvitelli", Department of Precision Medicine, Naples, Italy.
- 1434 Université Toulouse III, Toulouse, France.
- 1435 Sapporo Medical University, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo, Japan.
- 1436 University of California San Diego, Department of Pharmacology, La Jolla, CA, US.
- 1437 University of Colorado Denver, Department of Medicine, Aurora, CO, USA.
- 1438 Health Sciences University of Hokkaido, College of Rehabilitation Sciences, Department of Physical Therapy, Ishikari-Tobetsu, Hokkaido, Japan.
- 1439 Nagasaki University Graduate School of Biomedical Sciences, Department of Infectious Diseases, Nagasaki, Japan.
- 1440 Tokyo Medical University, Department of Biochemistry, Tokyo, Japan.
- 1441 Aarhus University, Department of Biomedicine, Aarhus, Denmark.
- 1442 Université Côte d'Azur (UCA), Institute for Research on Cancer and Aging of Nice (IRCAN), Centre Antoine Lacassagne, Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), Fédération Hospitalo-Universitaire (FHU) OncoAge, Nice, France.
- 1443 Kerman University of Medical Sciences, Institute of Neuropharmacology, Pharmaceutics Research Center, Kerman, Iran.
- 1444 MVR Cancer Hospital and Research Institute, Molecular Oncology, Cancer Genomics/Proteomics, Kerala, India.
- 1445 University of Kaiserslautern, Department of Biology, Plant Physiology, Kaiserslautern, Germany.
- 1446 International Centre for Genetic Engineering and Biotechnology, New Delhi, India.
- 1447 Department of Experimental Pathology, Institute of Biomedical Research of Barcelona, Spanish Research Council (IIBB-CSIC); IDIBAPS and CIBEREHD, Barcelona, Spain.
- 1448 Washington University in St. Louis, Professor of Obstetrics and Gynecology, St. Louis, MO, USA.
- 1449 Università della Svizzera italiana (USI), Faculty of Biomedical Sciences, Institute for Research in Biomedicine, Bellinzona, Switzerland; School of Life Sciences, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.
- 1450 University "Magna Graecia" of Catanzaro, Department of Health Sciences, Catanzaro, Italy.
- 1451 Aarhus University Hospital, Steno Diabetes Center Aarhus, Aarhus, Denmark; Aarhus University, Department of Clinical Medicine, Research Laboratory for Biochemical Pathology, Aarhus, Denmark.
- 1452 Université de Lyon, ENSL, UCBL, CNRS, LBMC, UMS 3444 Biosciences Lyon Gerland, Lyon, France.
- 1453 Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Científicas, Department of Molecular Biomedicine, Laboratory of Cell Death and Cancer Therapy, Madrid, Spain.
- 1454 Bispebjerg Hospital, Institute of Sports Medicine Copenhagen, Copenhagen,Denmark; UniCamillus, Saint Camillus International University of Health Sciences, Rome, Italy.
- 1455 University of Maryland School of Medicine, Center for Biomedical Engineering and Technology, Baltimore, MD, USA.
- 1456 Università degli Studi di Sassari, Dipartimento di Scienze Biomediche, Sassari, Italy.
- 1457 Centro di Riferimento Oncologico di Aviano (CRO), IRCCS, Immunopathology and Cancer Biomarkers, Aviano, Italy.
- 1458 University of Virginia, Department of Biology, Charlottesville, VA, USA.
- 1459 University of Exeter Medical School, European Centre for Environment & Human Health (ECEHH), Knowledge Spa, Royal Cornwall Hospital, Truro, Cornwall, UK; Plymouth Marine Laboratory (PML), Plymouth, UK; University of Plymouth, School of Biological & Marine Sciences, Plymouth, UK.
- 1460 National Institute of Genetic Engineering and Biotechnology, Institute of Medical Biotechnology, Molecular Medicine Department, Tehran, Iran.
- 1461 University of Pittsburgh, Department of Medicine, Aging Institute, Pittsburgh, PA, USA.
- 1462 Fondazione IRCCS Istituto Neurologico Carlo Besta, Neuromuscular Diseases and Neuroimmunology Unit, Milano, Italy.
- 1463 University of Coimbra, Institute of Physiology, Faculty of Medicine, Coimbra, Portugal and CNC - Center for Neuroscience and Cell Biology, Coimbra, Portugal.
- 1464 European Institute of Oncology (IEO) IRCCS, Department of Experimental Oncology, Milan, Italy.
- 1465 Laboratory of Neurodevelopment, Neurogenetics and Molecular Neurobiology, IRCCS Fondazione Santa Lucia, Rome, Italy.
- 1466 National Autonomous University of Mexico (UNAM), Department of Neurodevelopment and Physiology, Institute of Cellular Physiology, Mexico City, Mexico.
- 1467 Sapienza University of Rome, Department of Anatomy, Histology, Forensic Medicine & Orthopedics, Histology & Medical Embryology Section, Rome, Italy.
- 1468 Ifremer, SG2M-LGPMM, Laboratoire de Génétique et Pathologie des Mollusques Marins, La Tremblade, France.
- 1469 Swansea University Medical School, Molecular Neurobiology group, Swansea, United Kingdom.
- 1470 Normandie University, UNIROUEN, INSERM U1239, DC2N, Rouen, France; Institute for Research and Innovation in Biomedicine (IRIB), Rouen, France.
- 1471 Juntendo University Graduate School of Medicine, Department of Physiology, Tokyo, Japan.
- 1472 University of British Columbia, Department of Ophthalmology and Visual Sciences, Vancouver, British Columbia, Canada.
- 1473 Kindai University, Pharmaceutical Research and Technology Institute, Higashi-Osaka, Osaka, Japan.
- 1474 Saitama University, Department of Regulatory Biology, Saitama, Japan.
- 1475 Pontificia Universidad Católica de Chile, Faculty of Biological Sciences, Department of Physiology, Santiago de Chile, Chile.
- 1476 The University of Texas McGovern Medical School at Houston, Department of Neurology, Houston, TX, USA.
- 1477 Weill Cornell Medicine, Rockefeller University Campus, Department of Pathology and Laboratory Medicine, New York, NY, USA.
- 1478 Max Planck Institute for the Biology of Ageing, Cologne, Germany.
- 1479 Federal University of Ceara, Drug Research and Development Center, Fortaleza, CE, Brazil.
- 1480 Texas Tech University, Department of Nutritional Sciences & Obesity Research Institute, Lubbock, TX, USA.
- 1481 University of Münster, Institute of Medical Microbiology, Münster, Germany.
- 1482 Indian Council of Medical Research - National AIDS Research Institute, Division of Molecular Virology, Pune, MH, India.
- 1483 New York University Medical School, Laura and Isaac Perlmutter Cancer Center, Department of Radiation Oncology, New York, NY, USA.
- 1484 University of Colorado Denver, Children's Hospital Colorado, Morgan Adams Foundation Pediatric Brain Tumor Research Program, Aurora, CO.
- 1485 CNRS Biotechnology and cell signaling, Ecole Supérieure de Biotechnologie de Strasbourg, Strasbourg University/Laboratory of excellence Medalis; University of Strasbourg Institute for Advanced Study, Strasbourg, France.
- 1486 ICREA, Pompeu Fabra University (UPF), Department of Experimental and Health Sciences, Ciberned, Barcelona, Spain; Spanish National Cardiovascular Research Center (CNIC), Madrid, Spain.
- 1487 National Hospital for Paraplagics (Sescam) Research Unit, Molecular Neuroprotection Group, Toledo, Spain.
- 1488 University of Zürich, Institute of Experimental Immunology, Viral Immunobiology, Zürich, Switzerland.
- 1489 King's College London, School of Cardiovascular Medicine and Sciences, London, UK.
- 1490 Old Dominion University, Frank Reidy Research Center for Bioelectrics, Norfolk, VA, USA.
- 1491 Royal College of Surgeons in Ireland, Department of Physiology & Medical Physics, Dublin 2, Ireland.
- 1492 University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada.
- 1493 Genentech, Inc. Department of Cancer Immunology, South San Francisco, CA, USA.
- 1494 University of Helsinki, Faculty of Pharmacy, Division of Pharmacology and Pharmacotherapy/Drug Research Program, Helsinki, Finland.
- 1495 Washington University School of Medicine, Department of Obstetrics and Gynecology, and Department of Pathology and Immunology, St. Louis, MO, USA.
- 1496 University of Rzeszow, Institute of Biology and Biotechnology, Department of Biotechnology, Rzeszow, Poland.
- 1497 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, P.O. Box 19395-5487, Iran.
- 1498 Cornell University, Department of Entomology, Ithaca, NY, USA.
- 1499 Rutgers-New Jersey Medical School, Department of Cell Biology and Molecular Medicine, Newark, NJ, USA.
- 1500 University Hospital of Lausanne, Central Laboratory of Hematology, Lausanne, Switzerland.
- 1501 Kyoto University, Department of Microbiology, Graduate School of Medicine, Kyoto, Japan.
- 1502 Gladstone Institute of Neurological Disease and University of California, San Francisco, Department of Neurology, San Francisco, CA, USA.
- 1503 Tokyo Institute of Technology, School of Life Science and Technology, Yokohama, Japan.
- 1504 University of South Florida, Department of Cell Biology, Microbiology, and Molecular Biology, Tampa, FL, USA.
- 1505 "Sapienza" University of Rome, Department of Clinical and Molecular Medicine, Laboratory affiliated to Istituto Pasteur Italia Fondazione Cenci Bolognetti, Rome, Italy.
- 1506 University of Cambridge, Cancer Research UK Cambridge Institute, Cambridge, UK.
- 1507 Soroka University Medical Center, Institute of Hematology and Ben-Gurion University of the Negev, Faculty of Health Sciences, Department of Clinical Biochemistry and Pharmacology, Beer-Sheva, Israel.
- 1508 Niigata University, Brain Research Institute, Department of Neurosurgery, Niigata, Japan.
- 1509 Nelson Mandela University, Department of Biochemistry and Microbiology, Port Elizabeth, South Africa.
- 1510 Ecole Polytechnique Fédérale de Lausanne (EPFL), Institute of Bioengineering (IBI) and Swiss Institute for Experimental Cancer Research (ISREC), Laboratory of Regenerative Hematopoiesis, Lausanne, Switzerland; Centre Hospitalier Universitaire Vaudois (CHUV), Departments of Oncology and Laboratory Medicine, Hematology Service, Lausanne, Switzerland.
- 1511 University of Arizona, Department of Medicine, Tucson, AZ, USA.
- 1512 Georgia State University, Department of Biology, Atlanta, GA, USA.
- 1513 University of Copenhagen, Novo Nordisk Foundation Center for Basic Metabolic Research, Copenhagen, Denmark.
- 1514 Radboud University Medical Center, Department of Internal Medicine, Nijmegen, The Netherlands.
- 1515 Maastricht University Medical Center, CARIM School for Cardiovascular Diseases, Department of Pathology, Maastricht, The Netherlands.
- 1516 University Hospital Basel, Department of Biomedicine, Ocular Pharmacology and Physiology, Basel, Switzerland.
- 1517 Karolinska Institutet, Department of Women's and Children's Health, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Solna, Sweden.
- 1518 King's College London GKT School of Medical Education, London, UK.
- 1519 The University of Sydney, Kolling Institute, Renal Medicine, Sydney, New South Wales, Australia.
- 1520 University of Kansas Medical Center. Department of Pharmacology, Toxicology and Thereapeutics, Kansas City, KS, USA.
- 1521 Trinity College Dublin, Department of Clinical Medicine, Trinity Centre for Health Sciences, Dublin, Ireland.
- 1522 Army Medical University (Third Military Medical University), Daping Hospital, Center of Bone Metabolism and Repair, State Key Laboratory of Trauma, Burns and Combined Injury, Trauma Center, Research Institute of Surgery, Laboratory for the Prevention and Rehabilitation of Military Training Related Injuries, Chongqing, China.
- 1523 University of Ferrara, Department of Chemical and Pharmaceutical Sciences, Ferrara, Italy.
- 1524 National Hospital for Paraplegics (SESCAM), Research Unit, Molecular Neuroprotection Group, Toledo, Spain.
- 1525 East Tennessee State University, Quillen College of Medicine, Center of Excellence for Inflammation, Infectious Diseases, and Immunity, Department of Medicine, Johnson City, TN, USA.
- 1526 ICMR-Vector Control Research Center, Unit of Microbiology and Immunology, Puducherry, India.
- 1527 University of Kansas School of Medicine, Department of Anatomy and Cell Biology, Kansas City, KS, USA.
- 1528 New York University School of Medicine, Departments of Psychiatry and Cell Biology, and the Nathan Kline Institute, Orangeburg, NY, USA.
- 1529 Faculdade de Medicina e Ciências Biomédicas, Universidade do Algarve, Faro, Portugal; ABC-RI, Algarve Biomedical Center Research Institute, Faro, Portugal; Centre for Biomedical Research, Faro, Portugal.
- 1530 Duke University, Department of Biology and Howard Hughes Medical Institute, Durham, NC, USA.
- 1531 University of Split, School of Medicine, Split, Croatia.
- 1532 Doshisha University Graduate School of Brain Science, Laboratory of Structural Neuropathology, Kyoto, Japan.
- 1533 Center for Molecular Biology of Heidelberg University (ZMBH) and German Cancer Research Center (DKFZ), DKFZ-ZMBH Alliance, Heidelberg, Germany.
- 1534 Danish Cancer Society Research Center, Unit for Cell Death and Metabolism, Center for Autophagy, Recycling and Disease, Copenhagen, Denmark; University of Copenhagen, Department of Cellular and Molecular Medicine, Faculty of Health Sciences, Copenhagen N, Denmark.
- 1535 Trinity College Dublin, Trinity Translational Medicine Institute, Dublin, Ireland.
- 1536 David Geffen School of Medicine at UCLA, Department of Microbiology, Immunology, and Molecular Genetics, Los Angeles, CA, USA.
- 1537 University of Messina, Sicily, Italy.
- 1538 Hopp Children's Cancer Center Heidelberg (KiTZ), Heidelberg, Germany; German Cancer Research Center (DKFZ), and German Cancer Consortium (DKTK), Clinical Cooperation Unit Pediatric Oncology, Im Neuenheimer Feld, Heidelberg, Germany.
- 1539 National Institute of Infectious Diseases, Department of Bacteriology I, Toyama, Tokyo, Japan.
- 1540 Université de Paris, Centre de recherche sur l'inflammation, Team Gut Inflammation, Inserm, U1149, CNRS, ERL8252, Paris, France.
- 1541 University of Iceland, Faculty of Medicine, Biomedical center, Reykjavik, Iceland.
- 1542 Hollings Cancer Center, Department of Biochemistry and Molecular Biology, Charleston, SC, USA.
- 1543 Ewha Womans University, Department of Life Science, Immune and Vascular Cell Network Research Center, National Creative Initiatives, Seoul, Korea.
- 1544 Chosun University, School of Medicine, Gwangju, Korea.
- 1545 Yonsei University, Wonju, Korea.
- 1546 Yamaguchi University, Joint Faculty of Veterinary Medicine, Laboratory of Veterinary Pharmacology, Yoshida, Yamaguchi, Japan.
- 1547 Hyogo College of Medicine, Department of Genetics, Hyogo, Japan.
- 1548 University of Perugia, Department of Medicine and Surgery, Perugia, Italy.
- 1549 Stanford University, School of Medicine, Department of Pathology, CA, USA.
- 1550 Osaka University, Laboratory of Mitochondrial Dynamics, Graduate School of Frontier Biosciences, Osaka, Japan.
- 1551 Tokyo Medical and Dental University, Tokyo, Japan.
- 1552 Kyoto University of Advanced Science, Faculty of Bioenvironmental Science, Kameoka, Kyoto, Japan.
- 1553 University of Porto, REQUIMTE/UCIBIO, Faculty of Pharmacy, Porto, Portugal.
- 1554 Amgen Inc., South San Francisco, CA, USA.
- 1555 University of California, Berkeley, Departments of Molecular and Cell Biology and Nutritional Sciences and Toxicology, Berkeley, CA, USA; Chan Zuckerberg Biohub, San Francisco, CA, USA.
- 1556 Rutgers University, Center for Advanced Biotechnology and Medicine, Piscataway, NJ, USA.
- 1557 University of Defense, Faculty of Military Health Sciences, Department of Radiobiology, Hradec Kralove, Czech Republic.
- 1558 The Chinese University of Hong Kong, Lui Che Woo Institute of Innovative Medicine, Centre for Cardiovascular Genomics and Medicine (CCGM), Shatin, Hong Kong, China; The Chinese University of Hong Kong, Faculty of Medicine, Department of Medicine & Therapeutics, Shatin, Hong Kong, China; Hong Kong Hub of Paediatric Excellence (HK HOPE), Hong Kong Children's Hospital, Kowloon, Hong Kong, China; Xiamen University, Xiamen Cardiovascular Hospital, Institute for Translational Medicine, Xiamen, China; Chinese Academy of Sciences, Kunming Institute of Zoology - The Chinese University of Hong Kong (KIZ-CUHK) Joint Laboratory of Bioresources and Molecular Research of Common Diseases, Kunming, Yunnan, China.
- 1559 The University of Illinois at Chicago College of Medicine, Department of Pharmacology, Chicago, IL, USA.
- 1560 Pontificia Universidad Católica de Chile, Escuela de Medicina and Centro Interdisciplinario de Neurociencias, Facultad de Medicina, Departamento de Neurología, Santiago, Chile.
- 1561 University of Pennsylvania Perelman School of Medicine and Division of Neurology, The Children's Hospital of Philadelphia, Department of Neurology, Philadelphia, PA, USA.
- 1562 Suez Canal University, Faculty of Veterinary Medicine, Ismailia, Egypt.
- 1563 University of Wisconsin-Madison, Center for Quantitative Cell Imaging and Department of Botany, Madison, WI, USA.
- 1564 German Institute of Human Nutrition Potsdam-Rehbruecke, Department of Molecular Toxicology, Nuthetal, Germany.
- 1565 Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy; Institute of Molecular Bioimaging and Physiology (IBFM), CNR, Milan, Italy.
- 1566 University of Southern California, Keck School of Medicine, Department of Molecular Microbiology and Immunology, Los Angeles, CA, USA.
- 1567 University Medical Center Göttingen, Center for Biostructural Imaging of Neurodegeneration, Department of Experimental Neurodegeneration, Göttingen, Germany; Max Planck Institute for Experimental Medicine, Göttingen, Germany; Newcastle University, The Medical School, Institute of Neuroscience, Newcastle upon Tyne, UK.
- 1568 Oslo University Hospital and University of Oslo, Department of Pathology, Tumor Immunology Lab, Oslo, Norway.
- 1569 Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Medical Biology Department, Istanbul, Turkey.
- 1570 Texas Biomedical Research Institute, Host-Pathogen Interactions Program, San Antonio, TX, USA.
- 1571 Baylor College of Medicine, Department of Molecular and Human Genetics, Houston, TX, USA.
- 1572 University of Naples Federico II, Department of Molecular Medicine and Medical Biotechnology, Naples, Italy.
- 1573 Université de Paris, Centre de Recherche des Cordeliers, Paris, France.
- 1574 Fondazione IRCCS Ospedale San Raffaele, Division of Neuroscience, Milan, Italy.
- 1575 University of São Paulo, Department of Parasitology, São Paulo, Brazil.
- 1576 University of Rome Tor Vergata, Department of Clinical Sciences and Translational Medicine, Rome, Italy.
- 1577 Goethe University Frankfurt, Buchmann Institute for Molecular Life Sciences (BMLS), Frankfurt am Main, Germany.
- 1578 Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, China.
- 1579 Affiliated Hospital of GuangDong Medical University, Key Laboratory of Prevention and Management of Chronic Kidney Disease of Zhanjiang City, Zhanjiang, Guangdong, China.
- 1580 Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
- 1581 National Center of Biomedical Analysis, Beijing, China.
- 1582 The Children's Hospital of Philadelphia, Center for Applied Genomics, Philadelphia, PA, USA.
- 1583 University of Pittsburgh Medical Center, Department of Pediatrics, Pittsburgh, PA, USA.
- 1584 University of Alberta, Department of Biochemistry, Edmonton, Canada.
- 1585 University of Salento, Department of Biological and Environmental Sciences and Technologies (Di.S.Te.B.A.), Lecce, Italy.
- 1586 Danish Cancer Society Research Center, Computational Biology Laboratory, Copenhagen, Denmark.
- 1587 Max Perutz Labs, University of Vienna, Vienna Biocenter (VBC), Vienna, Austria.
- 1588 Quadram Institute Bioscience, Department of Gut, Microbes and Health, Norwich, UK.
- 1589 Waksman Institute, Department of Genetics, Rutgers University, Piscataway, NJ, USA.
- 1590 Dong-A University Medical School, Department of Molecular Neuroscience, Busan, Korea.
- 1591 Medical College of Wisconsin, Department of Biochemistry, Milwaukee, WI, USA.
- 1592 Incheon National University, Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon, Korea.
- 1593 Yonsei University, Division of Biological Science and Technology, Wonju, Korea.
- 1594 Chonnam National University, The Future Life & Society Research Center, Gwangju, Korea.
- 1595 Chonbuk National University, Department of VeterinaryMedicine, Iksan, Korea.
- 1596 Ben Gurion University of the Negev, Department of Chemistry, Be'er Sheva, Israel.
- 1597 KU Leuven, Department of Cellular and Molecular Medicine & Leuven Kanker Instituut, Leuven, Belgium.
- 1598 Sanofi, Vitry Sur Seine, France.
- 1599 Mayo Clinic, Department of Physiology and Biomedical Engineering, Rochester, MN, USA.
- 1600 VA San Diego Healthcare Systems and University of California, San Diego, Department of Anesthesiology, La Jolla, CA, USA.
- 1601 Internal Medicine 1, Brandenburg Hospital, Faculty of Health Sciences, Joint Faculty of the Brandenburg University of Technology Cottbus Senftenberg, the Brandenburg Medical School Theodor Fontane and the University of Potsdam, Brandenburg, Germany.
- 1602 IRCM, Institut de Recherche en Cancérologie de Montpellier, Tumor Microenvironment and Resistance to Treatment Laboratory, INSERM U1194, Université de Montpellier, Institut régional du Cancer de Montpellier, Montpellier, France.
- 1603 Universidad Nacional Autónoma de México, Instituto de Biotecnología, Departamento de Medicina Molecular y Bioprocesos, Laboratorio de Neuroinmunobiología, Cuernavaca, Morelos, México.
- 1604 Universidad de Chile, School of Medicine, Instituto de Ciencias Biomédicas, Santiago de Chile, Chile.
- 1605 Friedrich-Loeffler-Institut, Insitute of Immunology, Greifswald - Insel Riem, Germany.
- 1606 The Fourth Military Medical University, National Key Discipline of Cell Biology, Department of Physiology and Pathophysiology, Xi'an, Shaanxi, China.
- 1607 Weizmann Institute of Science, Department of Plant and Environmental Sciences, Rehovot, Israel.
- 1608 Centro de Investigaciones Biológicas CSIC, Department of Cellular and Molecular Biology, Madrid, Spain.
- 1609 Sun Yat-Sen University, Sun Yat-sen Memorial Hospital, Department of Neurology, Guangzhou City, Guangdong, China.
- 1610 University of Padova, Department of Biomedical Sciences, Padova, Italy.
- 1611 Fondazione G. Pascale, Istituto Nazionale Tumori IRCCS, Cell Biology and Biotherapy Unit, Naples, Italy.
- 1612 University of Coimbra, CNC - Center for Neuroscience and Cell Biology & Faculty of Medicine & CIBB - Center for Innovative Biomedicine and Biotechnology, Coimbra, Portugal.
- 1613 Universidade Federal de São Paulo, Paulista School of Medicine, Departament of Pharmacology, São Paulo, Brazil.
- 1614 São Paulo State University, College of Agronomy Sciences, Department of Bioprocesses and Biotechnology, Center for Evaluation of Environmental Impact on Human Health (TOXICAM), Botucatu, SP, Brazil.
- 1615 University of Melbourne, Florey Institute of Neuroscience and Mental Health, Melbourne, VIC, Australia.
- 1616 University of Granada, Faculty of Pharmacy, Department of Physical Chemistry, Granada, Spain.
- 1617 University of Nebraska Medical Center, Department of Pharmacology and Experimental Neuroscience, Omaha, NE, USA.
- 1618 State University of New York, Upstate Medical University, Departments of Medicine, Microbiology and Immunology, Biochemistry and Molecular Biology, Syracuse, NY, USA.
- 1619 Università degli Studi di Milano, Department of Biomedical and Clinical Sciences "L. Sacco", Milan, Italy.
- 1620 University of Calabria, Department of Biology, Ecology and Earth Sciences, Centre for Microscopy and Microanalysis (CM2), Rende (CS), Calabria, Italy.
- 1621 Baruch S. Blumberg Institute, Pennsylvania Cancer and Regenerative Medicine Research Center, Wynnewood, PA, USA; Xavier University School of Medicine, Woodbury, NY, USA.
- 1622 National Jewish Health and the University of Colorado, Denver, CO, USA.
- 1623 University of Oslo, Oslo University Hospital and Institute for Clinical Medicine, Department of Ophthalmology, Center for Eye Research, Oslo, Norway.
- 1624 Health and Medical University, Potsdam, Germany.
- 1625 Ulm University, Institute of Biochemistry and Molecular Biology, Faculty of Medicine, Ulm, Germany.
- 1626 Washington University, Division of Infectious Diseases, Department of Medicine and Molecular Microbiology, St. Louis, MO, USA.
- 1627 Third Military Medical University, Department of Occupational Health, Chongqing, China.
- 1628 Virginia Polytechnic Institute and State University, School of Neuroscience, Blacksburg, VA, USA.
- 1629 Aix Marseille Université, CNRS, INSERM, CIML, 13288 Marseille cedex 9, France; Institute for Research in Biomedicine (iBiMED) and Ilidio Pinho Foundation, Department of Medical Sciences, University of Aveiro, 3810-193 Aveiro, Portugal; Shanghai Institute of Immunology, Department of Microbiology and Immunology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, PR China.
- 1630 Karolinska Institutet, Department of Biosciences and Nutrition, Huddinge, Sweden.
- 1631 Consejo Superior de Investigaciones Científicas (CSIC), Instituto de Biología Molecular y Celular del Cáncer (IBMCC), Centro de Investigación del Cáncer (CSIC-Universidad de Salamanca), Universidad de Salamanca, Campus Unamuno, Salamanca. Spain.
- 1632 Tel Aviv University, School of Neurobiology, Biochemistry and Biophysics, Tel Aviv, Israel.
- 1633 The University of Texas MD Anderson Cancer Center, Department of Leukemia, Houston, TX, USA.
- 1634 Radboud University Medical Center, Nijmegen, The Netherlands.
- 1635 Ruhr-Universität Bochum, Medizinische Fakultät, Biochemie Intrazellulärer Transportprozesse, Bochum, Germany.
- 1636 Achucarro Basque Center for Neuroscience, Department of Pharmacology, University of the Basque Country EHU/UPV, Leioa, Bizkaia, Spain.
- 1637 University of Cologne, Center for Biochemistry, Cologne, Germany.
- 1638 Moscow State University, A.N.Belozersky Institute of Physico-Chemical Biology, Laboratory of Structure and function of mitochondria, Moscow, Russia.
- 1639 UCL Queen Square Institute of Neurology, London, UK.
- 1640 Monash Biomedicine Discovery Institute and Monash University, Department of Anatomy and Developmental Biology, Melbourne, Victoria, Australia.
- 1641 Georg-August University Göttingen, Institute of Microbiology and Genetics, Department of Genetics of Eukaryotic Microorganisms, Göttingen, Germany.
- 1642 Buchmann Institute & Institute of Biochemistry II, Medical Faculty, Goethe University, Frankfurt, Germany.
- 1643 Cancer Research Center of Toulouse, UMR 1037 Inserm-university of Toulouse, Toulouse, France.
- 1644 Mahidol University, Department of Microbiology, Faculty of Science, Ratchathewi, Bangkok, Thailand.
- 1645 Erasmus University Medical Center, Department of Epidemiology; Division of Pharmacology, Department of Internal Medicine, Rotterdam, The Netherlands.
- 1646 The Hospital for Sick Children, Translational Medicine Program, Toronto, Canada.
- 1647 St. Jude Children's Research Hospital, Department of Cell and Molecular Biology, Memphis, TN, USA.
- 1648 University of Oxford, Nuffield Department of Women's and Reproductive Health, Oxford, UK.
- 1649 University of California, Davis, Department of Molecular and Cellular Biology, Davis, CA, USA.
- 1650 University of Iowa, Department of Biology, Aging Mind and Brain Initiative, Iowa City, IA, USA.
- 1651 Jagiellonian University, Department of Evolutionary Immunology, Faculty of Biology, Krakow, Poland.
- 1652 Temple University, Alzheimer's Center at Temple, Philadelphia, PA, USA.
- 1653 Eberhard Karls University Tübingen, Interfaculty Institute of Cell Biology, Tübingen, Germany.
- 1654 University of Cyprus, Department of Biological Sciences, Bioinformatics Research Laboratory, Nicosia, Cyprus.
- 1655 Lifelong Health, South Australian Health & Medical Research Institute, North Terrace, Adelaide, Australia.
- 1656 University of Wisconsin-Madison, Department of Medicine and Neuroscience, Madison, WI, USA.
- 1657 Dalhousie University, Department of Biochemistry and Molecular Biology, Dalhousie Medicine New Brunswick, Saint John, NB, Canada.
- 1658 National Center for Cell Science, Pune, India.
- 1659 CURML, University Center of Legal Medicine, Lausanne University Hospital, Lausanne, Switzerland.
- 1660 Chinese Academy of Sciences, Kunming Institute of Zoology, Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences, Kunming, Yunnan, China.
- 1661 Lanzhou University, School of Life Sciences, Lanzhou, China.
- 1662 Zhejiang University, College of Medicine, The First Affiliated Hospital, Malignant Lymphoma Diagnosis and Therapy Center, Hangzhou, Zhejiang, China.
- 1663 China Pharmaceutical University, School of Basic Medicine and Clinical Pharmacy, State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Carcinogenesis and Intervention, Nanjing, China.
- 1664 Sanofi, Biologics Research, 49 New York Ave, Framingham, MA, USA.
- 1665 University of Waterloo, Department of Kinesiology, Waterloo, Ontario, Canada.
- 1666 Instituto de Investigaciones Biomedicas en Retrovirus y Sida, Universidad de Buenos Aires-CONICET, Argentina.
- 1667 University of Pittsburgh, Department of Pathology; University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
- 1668 Dartmouth College, Department of Chemistry, Hanover, NH, USA.
- 1669 Boston University, Department of Pathology and Laboratory Medicine, Boston, MA, USA.
- 1670 Tehran University of Medical Sciences, Cancer Biology Research Center, Tehran, Iran.
- 1671 Penn State University College of Medicine, Department of Cellular and Molecular Physiology, Hershey, PA, USA.
- 1672 University of Alabama at Birmingham, Cardiac Aging & Redox Signaling Laboratory, Molecular and Cellular Pathology, Department of Pathology, Birmingham, AL, USA.
- 1673 Johann Wolfgang Goethe-University, Institute of Anatomy III- 'Cellular and Molecular Anatomy', Frankfurt, Germany.
- 1674 University of Birmingham, Institute of Metabolism and Systems Research, Birmingham, UK.
- 1675 MRC Laboratory of Molecular Biology, Division of Protein and Nucleic Acid Chemistry, Cambridge, UK.
- 1676 Indian Institute of Science, Department of Biochemistry, Bangalore, India.
- 1677 School of Medicine, the Southern University of Science and Technology, Shenzhen, China.
- 1678 Baylor College of Medicine, Department of Medicine, Houston, TX, USA.
- 1679 The University of Kansas Cancer Center, Division of Hematologic Malignancies and Cellular Therapeutics, Kansas City, KS, USA.
- 1680 All India Institute of Medical Sciences, Department of Biotechnology, New Delhi, India.
- 1681 University of Southampton, Clinical and Experimental Sciences, Faculty of Medicine, UK.
- 1682 Johns Hopkins University School of Medicine, Department of Otolaryngology/Head and Neck Surgery, Head and Neck Cancer Research Division, Baltimore, MD, USA.
- 1683 University of South Carolina School of Medicine, Department of Pathology, Microbiology, and Immunology, Columbia, SC, USA.
- 1684 Washington University School of Medicine, Department of Medicine, Cardiovascular Division,l and John Cochran VA Medical Center, St. Louis, MO, USA.
- 1685 Johns Hopkins University, Bloomberg School of Public Health, Biochemistry and Molecular Biology Department, Baltimore, MD, USA.
- 1686 Johannes Gutenberg University-Mainz, Institute of Developmental Biology and Neurobiology, Mainz, Germany.
- 1687 Heinrich Heine University Düsseldorf, Medical faculty, Institute of Biochemistry and Molecular Biology I, Düsseldorf, Germany.
- 1688 The University of Texas MD Anderson Cancer Center, Institute for Applied Cancer Science (IACS), Translational Research to Advance Therapeutics and Innovation in Oncology (TRACTION), Houston, TX, USA.
- 1689 Max Planck Institute of Psychiatry, Translational Research in Psychiatry, Munich, Germany.
- 1690 University of Vienna, Cell Imaging and Ultrastructure Research (CIUS), Vienna, Austria.
- 1691 Karolinska Institutet, Department of Laboratory Medicine, Division of Clinical Microbiology, Stockholm, Sweden.
- 1692 Wright State University, Department of Biochemistry & Molecular Biology, Dayton, OH. USA.
- 1693 University of Wyoming, College of Health Sciences, Laramie, WY, USA.
- 1694 Qingdao Agricultural University, College of Plant Health and Medicine, Qingdao, Shandong, China.
- 1695 Ifremer, RBE, Département Ressources, La Tremblade, France.
- 1696 University of California, Los Angeles, David Geffen School of Medicine, Department of Human Genetics, Los Angeles, CA, USA.
- 1697 Botucatu Medical School, São Paulo State University, Botucatu, SP, Brazil.
- 1698 Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France.
- 1699 The Ohio State University, College of Veterinary Medicine, Department of Veterinary Biosciences, Columbus, OH, USA.
- 1700 Thomas Jefferson University, Department of Orthopaedic Surgery and Graduate Program in Cell Biology and Regenerative Medicine, Philadelphia, PA, USA.
- 1701 Josep Carreras Leukaemia Research Institute, Barcelona, Spain.
- 1702 Democritus University of Thrace, First Department of Internal Medicine & Laboratory of Molecular Hematology, Alexandroupolis, Greece.
- 1703 Redox Signaling and Oxidative Stress group, Danish Cancer Society Research Center, Copenhagen, Denmark.
- 1704 University of Padua, Department of Biomedical Sciences, Padua, Italy.
- 1705 Middlesex University, Department of Natural Sciences, London, UK.
- 1706 INSERM U1065, Centre Méditerranéen de Médecine Moléculaire (C3M), Nice, France; Université de Nice Sophia Antipolis, UFR de Médecine, Nice, France.
- 1707 Baylor College of Medicine, Department of Molecular Physiology & Biophysics, Houston, TX, USA.
- 1708 Federal University of ABC (UFABC), Center for Natural and Human Sciences (CCNH), Santo André, SP, Brazil.
- 1709 University of Campinas (UNICAMP), School of Applied Science, Department of Sport Sciences, Limeira, Brazil.
- 1710 University of Barcelona, School of Medicine, Unit of Anatomy, Department of Pathology and Experimental Therapeutics, Barcelona, Spain; L'Hospitalet de Llobregat, Institut d'Investigació Biomèdica de Bellvitge -IDIBELL, Molecular Mechanisms and Experimental Therapy in Oncology Program (oncobell), Barcelona, Spain.
- 1711 Kansas City University of Medicine and Biosciences, Department of Basic Science, Kansas City, MO, USA.
- 1712 Goethe University Frankfurt, Institute of Pharmaceutical Chemistry, Frankfurt am Main, Germany.
- 1713 University of Gdansk, Faculty of Chemistry, Gdansk, Poland.
- 1714 Department of Medicine and Surgery, University of Perugia, Perugia Italy.
- 1715 Università degli Studi di Catania, Dipartimento di Chirurgia Generale e Specialità Medico-Chirurgiche, Catania, Italy.
- 1716 CONICET-Universidad Nacional de Cuyo, Instituto de Histología y Embriología de Mendoza, Mendoza, Argentina.
- 1717 INSERM U1151, Institut Necker Enfants-Malades (INEM), Team 8, Université Paris Descartes-Sorbonne-Paris Cité, Paris, France.
- 1718 Naples University, Department of Veterinary Medicine and Animal Productions, Naples, Italy.
- 1719 University of Würzburg, Institute of Pathology, Würzburg, Germany.
- 1720 Kiel University and University Hospital Schleswig-Holstein, Campus Kiel, Institute of Clinical Molecular Biology, Kiel, SH, Germany.
- 1721 Georgetown University, Department of Biology, Washington, DC, USA.
- 1722 Columbia University, Department of Pathology and Cell Biology, New York, NY, USA.
- 1723 University of Illinois, Department of Anesthesiology, Chicago, IL, USA.
- 1724 Normandy University, UNICAEN, INSERM, UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Institute Blood and Brain @Caen-Normandie (BB@C), GIP Cyceron, Caen, FRANCE.
- 1725 University of Sherbrooke, Division of Rheumatology, Department of Medicine, Sherbrooke, QC, Canada.
- 1726 Section on Eukaryotic DNA Replication, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD,USA.
- 1727 University of Seville. School of Pharmacy, Instituto de Biomedicina de Sevilla. Sevilla, Spain.
- 1728 University of Cambridge, Cambridge Institute for Medical Research, UK Dementia Research Institute and Department of Medical Genetics, Cambridge, UK.
- 1729 Lomonosov Moscow State University, Chemistry Department, Moscow, Russia.
- 1730 University Medical Center Eppendorf, Institute for Medical Microbiology, Hamburg, Germany.
- 1731 Charles University, Faculty of Medicine in Hradec Kralove, Department of Medical Biology and Genetics, Hradec Kralove, Czech Republic.
- 1732 Mannheim University of Applied Sciences, Faculty of Biotechnology, Mannheim, Germany.
- 1733 Fondazione IRCCS Istituto Neurologico Carlo Besta, Neuromuscular Disease and Immunology Unit, Milan, Italy; University of Brescia, Unit of Biology and Genetics, Department of Molecular and Translation Medicine, Brescia, Italy.
- 1734 Australian Regenerative Medicine Institute, Monash University, Clayton, Victoria, Australia.
- 1735 University of Ottawa, Department of Cellular and Molecular Medicine, Ottawa, ON, Canada.
- 1736 National Research Council, Institute of Food Sciences, Avellino, Italy.
- 1737 Stazione Zoologica "Anton Dohrn", Napoli, Italy.
- 1738 University of Calabria, Department of Pharmacy, Health and Nutritional Sciences, Cosenza, Italy.
- 1739 N.N. Blokhin National medical research center of oncology, Department of biomarkers and mechanisms of tumor angiogenesis, Moscow, Russia.
- 1740 Cancer Research UK Beatson Institute, Glasgow, UK.
- 1741 University of Seoul, Department of Life Science, Seoul, Korea.
- 1742 University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
- 1743 Concordia University, Department of Biology, Montreal, Quebec, Canada; McGill University, Department of Anatomy and Cell Biology, Montreal, Quebec, Canada.
- 1744 Ernst-Ruska Centre for Microscopy and Spectroscopy with Electrons (ER-C-3/StructuralBiology), Forschungszentrum Jülich, Jülich, Germany.
- 1745 Saitama University, Graduate School of Science and Engineering, Saitama, Japan.
- 1746 Rutgers New Jersey Medical School, Department of Cell Biology and Molecular Medicine, Newark, NJ, USA.
- 1747 Oregon State University, College of Pharmacy, Department of Pharmaceutical Sciences, Portland, OR, USA.
- 1748 Mashhad University of Medical Sciences, Pharmaceutical Technology Institute, Biotechnology Research Center, Mashhad, Iran.
- 1749 Boston Children's Hospital and Harvard Medical School, Boston, MA, USA.
- 1750 University of South Carolina, Department of Drug Discovery and Biomedical Sciences, Columbia, SC, USA.
- 1751 University of Sydney, Northern Clinical School, Kolling Institute of Medical Research, Sydney, NSW, Australia.
- 1752 Institute for Research in Biomedicine (IRB Barcelona), Cellular Plasticity and Disease Group, Barcelona, Spain.
- 1753 University of Toyama, Sugitani, Toyama, Japan.
- 1754 Kyoto University, Graduate School of Agriculture, Division of Applied Life Sciences, Kyoto, Japan.
- 1755 The University of Tokyo, Graduate School of Medicine, Department of Biochemistry and Molecular Biology, Tokyo, Japan.
- 1756 University of Helsinki and Helsinki University Hospital, Department of Virology, Helsinki, Finland.
- 1757 Florida State University, Department of Nutrition, Food and Exercise Sciences, Tallahassee, FL, USA.
- 1758 Institut Hospital del Mar d'Investigacions Mèdiques (IMIM), Research Group in Critical Disorders (GREPAC), Barcelona, Spain.
- 1759 Agricultural Biotechnology Research Institute of Iran (ABRII), Department of Systems Biology, Karaj, Iran.
- 1760 University of Minho, School of Medicine, Life and Health Sciences Research Institute (ICVS), Braga, Portugal; ICVS/3B's - PT Government Associate Laboratory, Braga/Guimarães, Portugal.
- 1761 CABD/CSIC/Universidad Pablo de Olavide, Departamento de Fisiología, Anatomía y Biología Celular, Sevilla, Spain.
- 1762 University of Malaga, Department of Molecular Biology and Biochemistry, Malaga, Spain.
- 1763 Imperial College London, Section of Paediatric Infectious Disease & Virology, London, UK.
- 1764 INRS-Institut Armand-Frappier, Laval, QC, Canada.
- 1765 Weill-Cornell, Englander Institute of Precision Medicine, Department of Radiation Oncology, New York, NY, USA.
- 1766 Centre for Innovative Biomedicine and Biotechnology, University of Coimbra, Coimbra, Portugal.
- 1767 University of Camerino, School of Pharmacy, Experimental Medicine Section, Camerino, Italy.
- 1768 University of Glasgow, Institute of Molecular, Cell and Systems Biology, Glasgow, Scotland, United Kingdom.
- 1769 Instituto de Biomedicina de Valencia, CSIC, Valencia, Spain.
- 1770 Jawaharlal Nehru University, School of Life Sciences, New Delhi, India.
- 1771 Institute for Stem Cell Science & Regenerative Medicine (inStem), Bangalore, Karnataka, India.
- 1772 University of Maryland School of Medicine, Department of Anesthesiology, Shock, Trauma and Anesthesiology Research Center, Baltimore, MD, USA.
- 1773 University of Birmingham, Institute of Biomedical Research, Institute of Cancer and Genomic Sciences, College of Medical and Dental Sciences, Edgbaston, Birmingham, UK.
- 1774 Health Research Institute Germans Trias i Pujol, Innate Immunity Group, Badalona, Barcelona, Spain.
- 1775 University of Delhi South Campus, Department of Genetics, New Delhi, India.
- 1776 Folkhälsan Research Center, Helsinki, Finland.
- 1777 Mayo Clinic, Department of Molecular Medicine, Rochester, MN, USA.
- 1778 National Institute of Mental Health and Neurosciences, Department of Neurochemistry, Bengaluru, Karnataka, India.
- 1779 Duke University, Department of Molecular Genetics and Microbiology, Durham, NC, USA.
- 1780 Goethe University, Institute of Pharmacology and Toxicology, Frankfurt, Germany.
- 1781 University of Notre Dame, Department of Biological Sciences, Notre Dame, IN ,USA.
- 1782 UCL Queen Square Institute of Neurology, Department of Clinical and Movement Neurosciences, London, UK.
- 1783 University Hospital and University of Zürich, Department of Gastroenterology and Hepatology, Zürich, Switzerland.
- 1784 University of Calgary, Department of Comparative Biology & Experimental Medicine, Calgary, AB, Canada.
- 1785 Department of Biochemistry and Molecular Biology, Institute for Human Infection and Immunity (IHII), University of Texas Medical Branch at Galveston, Galveston, TX, USA.
- 1786 Amsterdam University Medical Centers location VUmc, Department of Clinical Genetics, Amsterdam, The Netherlands.
- 1787 University of Kaiserslautern, Phytopathology, Kaiserslautern, Germany.
- 1788 IRCCS San Raffaele Scientific Institute, Division of Genetics and Cell Biology, Milan, Italy.
- 1789 Centro di Riferimento Oncologico, CRO-IRCCS, Molecular Oncology Unit, Aviano, Italy.
- 1790 Medical University of Graz, Gottfried Schatz research center, Graz, Austria.
- 1791 University Grenoble Alpes and Inserm U1055, Laboratory of Fundamental and Applied Bioenergetics (LBFA), Grenoble, France.
- 1792 Medical University of Innsbruck, Institute for Cell Biology, Innsbruck, Austria.
- 1793 Hannover Medical School, Department of Nephrology and Hypertension, Hannover, Germany.
- 1794 National Institutes of Health, National Institute of Allergy and Infectious Disease, Vaccine Research Center, Bethesda, MD, USA.
- 1795 Ruhr-University Bochum, Department of Molecular Immunology, Bochum, Germany.
- 1796 German Center for Neurodegenerative Diseases, DZNE, Bonn, Germany and Department of Neurodegenerative Diseases and Geriatric Psychiatry, University Bonn, Bonn, Germany.
- 1797 Reseach Center Borstel, Leibniz Lung Center, Priority Research Area Infections, Junior Research Group Coinfection, Borstel, Germany.
- 1798 Johannes Kepler University Linz, Institute of Biophysics, Linz, Austria.
- 1799 Instituto de Investigaciones en Ingeniería Genética y Biología Molecular Dr. Héctor N. Torres, Laboratorio de Señalización y Mecanismos Adaptativos en Tripanosomátidos, Buenos Aires, Argentina; Universidad de Buenos Aires, Departamento de Fisiología, Biología Molecular y Celular, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina.
- 1800 Instituto de Investigaciones en Ingeniería Genética y Biología Molecular Dr. Héctor N. Torres, Laboratorio de señalización y mecanismos adaptativos en Tripanosomátidos, Buenos Aires, Argentina; Universidad de Buenos Aires, Departamento de Química Biológica, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina.
- 1801 Mayo Clinic, Department of Biochemistry and Molecular Biology, Division of Gastroenterology and Hepatology, Rochester, MN, USA.
- 1802 Technische Universität Dresden, Institute for Physiological Chemistry, Dresden, Germany.
- 1803 University of Wuerzburg, Department of Vegetative Physiology, Wuerzburg, Germany.
- 1804 University of Natural Resources and Life Sciences, Department of Applied Genetics and Cell Biology, Vienna, Austria.
- 1805 Mayo Clinic, Division of Gastroenterology and Hepatology, Rochester, MN, USA.
- 1806 University of Luxembourg, Luxembourg Centre for Systems Biomedicine (LCSB), Esch-sur-Alzette, Luxembourg.
- 1807 Forschungszentrum Jülich, Institute of Biological Information Processing, IBI-7 (Structural Biochemistry), Jülich, Germany.
- 1808 University of Campania L. Vanvitelli, Dipartimento di Scienze Mediche Traslazionali, Naples, Italy.
- 1809 Department of Medical and Surgical Sciences and Biotechnologies, Sapienza University of Rome, Italy; IRCCS Neuromed, Pozzilli (IS), Italy.
- 1810 Rutgers Biomedical and Health Sciences, Rutgers University, Robert Wood Johnson Medical School, Department of Pharmacology, New Brunswick , NJ, USA.
- 1811 National Research Council, Institute of Molecular Genetics, Pavia, Italy.
- 1812 Helmholtz Centre for Infection Research, Braunschweig, Germany.
- 1813 Albert Einstein College of Medicine, Department of Developmental and Molecular Biology, Bronx, NY, USA; Albert Einstein College of Medicine, Institute for Aging Studies, Bronx, NY, USA.
- 1814 Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Barcelona, Spain; Departament de Bioquímica i Biomedicina Molecular, Facultat de Biologia, Universitat de Barcelona, Barcelona, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
- 1815 Rice University, Department of Bioengineering, Houston, TX, USA.
- 1816 University of Illinois at Chicago, College of Medicine, Department of Biochemistry and Molecular Genetics, Chicago, IL, USA.
- 1817 University of Oslo, Centre for Molecular Medicine Norway (NCMM), Oslo, Norway.
- 1818 Université de Pau et des Pays de l'Adour, E2S UPPA, INRAE, UMR1419 Nutrition Métabolisme et Aquaculture, F-64310 Saint-Pée-sur-Nivelle, France.
- 1819 Cedars-Sinai Medical Center, Department of Medicine, Division of Digestive and Liver Diseases, Los Angeles, CA, USA.
- 1820 Pennsylvania State University, Department of Biochemistry and Molecular Biology, University Park, PA, USA.
- 1821 Alpert Medical School of Brown University/Rhode Island Hospital, Division of Cardiothoracic Surgery, Providence, RI, USA.
- 1822 Iowa State University, Animal Science Department, Ames, IA, USA.
- 1823 Dokuz Eylul University, Izmir International Biomedicine and Genome Institute, Izmir, Turkey.
- 1824 University of Alberta, Department of Laboratory Medicine and Pathology, Edmonton, AB, Canada.
- 1825 University of Rochester Medical Center, Microbiology and Immunology, Rochester, NY, USA.
- 1826 Johns Hopkins University School of Medicine, Department of Cell Biology, Baltimore, MD, USA.
- 1827 Indian Institute of Science, Department of Microbiology and Cell Biology, Bangalore, KA, India.
- 1828 Shenzhen University Health Science Center, School of Dentistry, Shenzhen, Guangdong Province, China.
- 1829 University of Alabama at Birmingham, Department of Pharmacology & Toxicology, Birmingham, AL, USA.
- 1830 University of Washington, Department of Medicine, Seattle, WA, USA; VA Puget Sound Health Care System, Seattle, WA, USA.
- 1831 Soochow University, Institutes for Translational Medicine, Suzhou, Jiangsu, China.
- 1832 Freie Universitaet Berlin, Department of Veterinary Medicine, Institute of Veterinary Biochemistry, Berlin, Germany.
- 1833 Johns Hopkins University, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, USA.
- 1834 University of Pennsylvania, Abramson Cancer Center, Department of Medicine, PA, USA.
- 1835 Division of Stem Cell and Gene Therapy Research, Institute of Nuclear Medicine & Allied Sciences (INMAS), Defence Research and Development Organization (DRDO), Timar Pur, New Delhi, India.
- 1836 Thomas Jefferson University, Center for Translational Medicine, Philadelphia, PA, USA.
- 1837 Women and Infants Hospital of Rhode Island-Warren Alpert Medical School of Brown University, Department of Pediatrics, Providence, RI, USA.
- 1838 University of Macau, Faculty of Health Sciences, Macau, China.
- 1839 The University of Hong Kong, School of Chinese Medicine, Hong Kong, China.
- 1840 Nanjing Agricultural University, College of Animal Science and Technology, Nanjing, Jiangsu, China.
- 1841 Shanghai Jiao Tong University School of Medicine, Department of Hypertension, Ruijin Hospital, Shanghai, China.
- 1842 Total Toxicology Labs, Southfield, MI, USA.
- 1843 Soochow University, College of Pharmaceutical Sciences, Department of Pharmacology and Laboratory of Aging and Nervous Diseases, Suzhou, China.
- 1844 Fralin Biomedical Research Institute at VTC, Roanoke, VA, USA.
- 1845 Indiana University School of Medicine, Department of Pediatrics, Indianapolis, IN, USA.
- 1846 Van Andel Institute, Center for Epigenetics, Grand Rapids, MI, USA.
- 1847 Fudan University, Zhongshan Hospital, Liver Cancer Institute, Department of Liver Surgery & Transplantation, Shanghai, China.
- 1848 Juntendo University Graduate School of Medicine, Department of Treatment and Research in Multiple Sclerosis and Neuro-intractable Disease, Bunkyo-ku, Tokyo, Japan.
- 1849 National Chung-Hsing University, Institute of Biomedical Sciences, Taichung City, Taiwan.
- 1850 Jikei University School of Medicine, Research Center for Medical Sciences, Division of Gene Therapy, Tokyo, Japan.
- 1851 Tokyo Medical and Dental University, Department of Pathological Cell Biology, Tokyo, Japan.
- 1852 Tohoku University, Graduate School of Agricultural Science, Sendai, Japan.
- 1853 Geisel School of Medicine at Dartmouth, Department of Biochemistry and Cell Biology, Hanover, NH, USA.
- 1854 Kobe University Graduate School of Medicine, Center for Infectious Diseases, Division of Infectious Disease Control, Kobe, Japan.
- 1855 MACS - Agharkar Research Institute, Developmental Biology Group, Pune, India; Savitribai Phule Pune University, Ganeshkhind, Pune, Maharashtra, India.
- 1856 Mayo Clinic College of Medicine, Department of Experimental Pathology, Rochester, MN, USA.
- 1857 National Sun Yat-sen University, Institute of Biopharmaceutical Sciences, Kaohsiung, Taiwan.
- 1858 Huazhong University of Science and Technology, College of Life Science and Technology, Key Laboratory of Molecular Biophysics of Ministry of Education,Wuhan, Hubei, China.
- 1859 University of Calgary, Cumming School of Medicine, Departments of Medical Genetics and Biochemistry & Molecular Biology, Calgary, Alberta, Canada.
- 1860 Department of Experimental and Health Sciences, Pompeu Fabra University (UPF), CIBER on Neurodegenerative diseases (CIBERNED), E-08003 Barcelona, Spain.
- 1861 Achucarro Basque Center for Neuroscience, Department of Neurosciences, University of the Basque Country EHU/UPV, Leioa, Bizkaia, Spain.
- 1862 Universidad de la República, Facultad de Agronomía, Departamento de Biología Vegetal, Montevideo, Uruguay.
- 1863 National Institutes of Health, NIEHS, RTP, Durham, NC, USA.
- 1864 David Geffen School of Medicine at University of California, Los Angeles, Department of Medicine, Division of Dermatology, Los Angeles, CA, USA.
- 1865 University of Bern, Institute of Pharmacology, Bern, Switzerland; Sechenov University, Department of Clinical Immunology and Allergology, Moscow, Russia.
- 1866 INSERM UMR1163, Laboratory of Epithelial Biology and Disease, Imagine Institute, Paris Descartes University, Sorbonne Paris Cité, Hôpital Necker-Enfants Malades, Paris, France.
- 1867 Boston University School of Medicine, Division of Hematology & Medical Oncology, Department of Pharmacology & Experimental Therapeutics, Boston, MA, USA.
- 1868 Wayne State University School of Medicine, Department of Ophthalmology, Visual and Anatomical Sciences, Detroit, MI, USA.
- 1869 University of Pittsburgh, Department of Pharmacology & Chemical Biology, Pittsburgh, PA, USA.
- 1870 Post Graduate Institute of Medical Education and Research (PGIMER), Department of Urology, Chandigarh, India.
- 1871 Biomedical Research Institute of New Mexico, VA Healthcare System, Albuquerque, NM, USA.
- 1872 CSIR-National Physical Laboratory, Dr. K. S. Krishnan Marg, New Delhi, India.
- 1873 University of Pittsburgh School of Medicine, Departments of Ophthalmology, Cell Biology, and Developmental Biology, Pittsburgh, PA, USA.
- 1874 Sanjay Gandhi Postgraduate Institute of Medical Sciences, Department of Endocrinology, Lucknow, India.
- 1875 North Dakota State University, Department of Chemistry and Biochemistry, Fargo, ND, USA.
- 1876 National Jewish Health, Department of Medicine, Division of Allergy & Immunology, and Division of Pulmonary and Critical Care Medicine, Denver, CO, USA.
- 1877 Masaryk University, Faculty of Medicine, Department of Biology, Brno, Czech Republic.
- 1878 Washington State University, Institute of Biological Chemistry, Pullman, WA, USA.
- 1879 Edinburgh Cancer Research UK Centre, MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK.
- 1880 Dong-A University, College of Medicine, Department of Molecular Neuroscience, Peripheral Neuropathy Research Center, Busan, Korea.
- 1881 Lomonosov Moscow State University, Department of Biology, Moscow, Russia.
- 1882 Indian Institute of Technology Indore, Discipline of Biosciences and Biomedical Engineering, Indore, Madhya Pradesh, India.
- 1883 Shandong University, School of public health, Department of Toxicology and nutrition. Jinan, Shandong, China.
- 1884 Korea University, Department of Life Sciences, Seoul, Korea.
- 1885 Guangzhou University of Chinese Medicine, Medical College of Acupuncture-Moxibustion and Rehabilitation, Guangzhou, China; Hong Kong Baptist University, School of Chinese Medicine, Mr. and Mrs. Ko Chi Ming Centre for Parkinson's Disease Research, Hong Kong SAR, China.
- 1886 University of Colorado Anschutz Medical Campus, Division of Cardiology, Aurora, CO, USA.
- 1887 Hubei Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan, Hubei, China.
- 1888 Massachusetts General Hospital and Harvard Medical School, Department of Medicine, Endocrine Division, Boston, MA, USA.
- 1889 University of Guelph, Department of Food Science, Guelph, Canada.
- 1890 University of California, San Diego, La Jolla, CA, USA; Rady Children's Hospital San Diego, San Diego, CA, USA.
- 1891 Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bengaluru-560029, Karnataka, India.
- 1892 University of Kentucky, Department of Toxicology and Cancer Biology, Lexington, KY, USA.
- 1893 Institute of Molecular Biology and Pathology, National Research Council (CNR), Rome, Italy; Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione Santa Lucia, Laboratory of Cell Signaling, Rome, Italy.
- 1894 University of Western Ontario, Department of Physiology and Pharmacology, London, Ontario, Canada.
- 1895 University of Oslo, Department of Neuro-/Pathology, Oslo, Norway.
- 1896 University of Bonn, Department of Pharmaceutical & Cellbiological Chemistry, Bonn, Germany.
- 1897 Biomedical Research Foundation of the Academy of Athens, Laboratory of Neurodegenerative Diseases, Athens, Greece; National and Kapodistrian University of Athens Medical School, First Department of Neurology, Athens, Greece.
- 1898 University of California, Irvine, Department of Psychiatry and Human Behavior, Irvine, CA, USA.
- 1899 Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Immune Modulation, University Hospital Erlangen, Erlangen, Germany.
- 1900 Oslo University Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Montebello, Oslo, Norway.
- 1901 Technische Universität Dresden, Center for Regenerative Therapies Dresden, Dresden, Germany.
- 1902 Jožef Stefan Institute, Department of Biochemistry and Molecular and Structural Biology, Ljubljana, Slovenia.
- 1903 University Medical Center Hamburg-Eppendorf, Children's Hospital, University Children's Research@Kinder-UKE, Hamburg, Germany.
- 1904 Heinrich-Heine-University, Medical Faculty, Institute of Molecular Medicine I, Düsseldorf, Germany.
- 1905 IRCCS Fondazione Santa Lucia, Rome, Italy.
- 1906 The Ohio State University, Department of Cancer Biology and Genetics, Columbus, OH, USA.
- 1907 University of California San Diego, UCSD Moores Cancer Center, La Jolla, CA, USA.
- 1908 Peking Union Medical College and Chinese Academy of Medical Sciences, Peking Union Medical College Hospital, Department of Critical Care Medicine, Beijing, China.
- 1909 Instituto Oswaldo Cruz, LITEB/IOC, Fundação Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, Brazil.
- 1910 Case Western Reserve University School of Medicine, Departments of Medicine and Pathology, Cleveland, OH USA.
- 1911 Rutgers University, New Jersey Medical School and Public Health Research Institute, Newark, NJ, USA.
- 1912 University of Madras, Department of Biochemistry, Guindy Campus, Chennai, India.
- 1913 Kolling Institute, Department of Neurogenetics, University of Sydney and Royal North Shore Hospital, St Leonards NSW, Australia.
- 1914 Hangzhou Normal University, Affiliated Hospital of Hangzhou Normal University, College of Medicine, Holistic Integrative Pharmacy Institutes and Comprehensive Cancer Diagnosis and Treatment Center, Hangzhou, Zhejiang, China.
- 1915 Iowa State University, Department of Kinesiology, Ames, IA, USA.
- 1916 University of Nebraska Lincoln, Department of Agronomy and Horticulture, Center for Plant Science Innovation, Lincoln, NE, USA.
- 1917 University of Illinois at Chicago, Department of Medicine, Chicago, IL, USA.
- 1918 Shanghai Jiao Tong University, School of Medicine, Ren Ji Hospital, Center for Reproductive Medicine, Shanghai, China.
- 1919 Wuhan University, College of Life Sciences, Wuhan, Hubei, China.
- 1920 Zhejiang University, School of Medicine, Department of Biochemistry, Hangzhou, Zhejiang, China.
- 1921 Cancer Institute of the Second Affiliated Hospital and Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
- 1922 University of Tennessee Health Science Center, Department of Physiology, Memphis, TN, USA.
- 1923 Experimental Physiopathology, Department of Sciences/Experimental Physiopatholgy, Medical School, University of São Paulo, Brazil.
- 1924 Swedish University of Agricultural Sciences, The Linnean Centre of Plant Biology in Uppsala, Department of Plant Biology, Uppsala, Sweden.
- 1925 University of Pennsylvania, Perelman School of Medicine, Department of Medicine, Philadelphia, PA, USA.
- 1926 University of Missouri, Division of Animal Science and Department of Obstetrics, Gynecology & Women's Health, Columbia, MO, USA.
- 1927 Tokai University School of Medicine, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Isehara, Kanagawa, Japan.
- 1928 York University, Department of Biology, Toronto, Canada.
- 1929 University of Utah, Department of Nutrition and Integrative Physiology; Division of Endocrinology, Metabolism, and Diabetes; Investigator, Molecular Medicine Program, Salt Lake City, UT, USA.
- 1930 Hong Kong Baptist University, Faculty of Science, Department of Biology, Hong Kong, China; Hong Kong Baptist University, Golden Meditech Centre for NeuroRegeneration Sciences, Hong Kong, China.
- 1931 Ohio Musculoskeletal and Neurological Institute (OMNI) and Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH, USA.
- 1932 Istituto Superiore di Sanità, Department of Oncology and Molecular Medicine, Rome, Italy; Fondazione Umberto Veronesi, Milan, Italy.
- 1933 Charité University Medicine Berlin, Department of Hepatology & Gastroenterology, Berlin, Germany.
- 1934 The University of Texas Health Science Center at Houston, McGovern Medical School, Department of Internal Medicine, Cardiology, Houston, TX, USA.
- 1935 Chengdu Medical College, Department of Anatomy and Histology and Embryology, Development and Regeneration Key Lab of Sichuan Province, Chengdu, China.
- 1936 University of Glasgow, Institute of Cancer Sciences, Glasgow, UK.
- 1937 Penn State University College of Medicine, Department of Pediatrics, Hershey, PA, USA.
- 1938 VIT University, School of Biosciences and Technology, Vellore, Tamilnadu, India.
- 1939 The Chinese University of Hong Kong, School of Biomedical Sciences, Hong Kong, China; The Chinese University of Hong Kong, Department of Obstetrics and Gynecology, Hong Kong, China.
- 1940 Inserm Unit 1195 - University of Paris Saclay, Le Kremlin-Bicetre, France.
- 1941 Tokyo Medical and Dental University (TMDU), Institute of Biomaterials and Bioengineering, Tokyo, Japan.
- 1942 National Neuroscience Institute, Duke NUS Medical School, Singapore.
- 1943 Key Laboratory of Oral Biomedicine Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, China.
- 1944 The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST), Wuhan University, School and Hospital of Stomatology, Department of Oral Medicine, Wuhan, Hubei, China.
- 1945 Kyoto Prefectural University of Medicine, Department of Anatomy and Neurobiology, Kyoto, Japan.
- 1946 RIKEN Center for Brain Science, Laboratory for Protein Conformation Diseases, Wako, Saitama, Japan.
- 1947 Hubei University of Technology, National "111" Center for Cellular Regulation and Molecular Pharmaceutics, Wuhan, China.
- 1948 State Key Laboratory of Membrane Biology, Institute of Zoology, Institute for Stem Cell and Regeneration, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Beijing, China.
- 1949 Juntendo University Graduate School of Medicine, Department of Cellular and Molecular Neuropathology, Tokyo, Japan.
- 1950 Harvard Medical School, Massachusetts General Hospital, Cutaneous Biology Research Center, Charlestown, MA, USA.
- 1951 University Medicine Goettingen, Neurology, Department of Translational Neurodegeneration, Center for Biostructural Imaging of Neurodegeneration (BIN), Goettingen, Germany.
- 1952 Foundation for Research and Technology-Hellas, Institute of Molecular Biology and Biotechnology, Heraklion, Crete, Greece; University of Crete, Medical School, Heraklion, Crete, Greece.
- 1953 Duke University, Department of Medicine; Duke University, Department of Molecular Genetics and Microbiology; Duke University, Department of Immunology, Durham, NC, USA; Geriatric Research, Education, and Clinical Center, VA Health Care Center, Durham, NC, USA.
- 1954 University of North Carolina, Department of Pathology, Chapel Hill, NC, USA.
- 1955 Nasonova Research Institute of Rheumatology, Moscow, Russia.
- 1956 Cardiff University, Division of Cancer and Genetics, Heath Park Way, Cardiff, UK.
- 1957 Goethe-University, Faculty of Medicine, Department of Clinical Pharmacology, Frankfurt, Germany.
- 1958 INSERM U955, Team "Virus Hepatology Cancers", Créteil, France; Université Paris-Est, UMR S955, UPEC, Créteil, France.
- 1959 Chulalongkorn University, Faculty of Allied Health Sciences, Age-Related Inflammation and Degeneration Research Unit, Department of Clinical Chemistry, Bangkok, Thailand.
- 1960 University of Liverpool, Department of Cellular and Molecular Physiology, Liverpool, UK.
- 1961 Centro de Investigaciones Biológicas Margarita Salas, CIB-CSIC, Madrid, Spain.
- 1962 Carl von Ossietzky University Oldenburg, School of Medicine and Health Sciences, Department for Neuroscience, Oldenburg, Germany.
- 1963 University of Genova, Department of Internal Medicine, Genova, Italy.
- 1964 University of Kansas Medical Center, Department of Otolaryngology, Kansas City, KS, USA.
- 1965 University of Nebraska Medical Center, Departments of Internal Medicine and Biochemistry and Molecular Biology, Omaha, NE, USA.
- 1966 University of Colorado School of Medicine, Department of Pharmacology, Aurora, CO, USA.
- 1967 CSIR-Institute of Genomics and Integrative Biology, South Campus, New Delhi, India.
- 1968 Hannover Medical School, Institute for Molecular and Therapeutic Strategies (IMTTS), Hannover, Germany.
- 1969 University Medical Center Göttingen, Institute of Cellular Biochemistry, Göttingen, Germany.
- 1970 South China Agricultural University, Guangdong Provincial Key Laboratory of Agro-animal Genomics and Molecular Breeding/Guangdong Provincial Sericulture and Mulberry Engineering Research Center, College of Animal Science, Guangzhou, China.
- 1971 University Hospital of Bonn, Institute of Reconstructive Neurobiology, Bonn, Germany.
- 1972 University of Antwerp, Department of Biomedical Sciences, Peripheral Neuropathy Research Group, Antwerp, Belgium.
- 1973 Concordia University, Department of Biology, Montreal, Quebec, Canada.
- 1974 Wayne State University School of Medicine, Department of Pharmacology, Detroit, MI, USA.
- 1975 University of Oviedo, Department of Functional Biology, Physiology, Oviedo, Asturias, Spain.
- 1976 Zhejiang University, Life Sciences Institute and Innovation Center for Cell Signaling Network, Hangzhou, China.
- 1977 Northwestern University, Feinberg School of Medicine, Department of Pathology, Chicago, IL, USA.
- 1978 Centre de Recherche des Cordeliers, INSERM, Université de Paris, Sorbonne Université, Paris, France.
- 1979 Nagoya University Graduate School of Medicine, Department of Pathology and Biological Responses, Nagoya, Japan.
- 1980 University of Belgrade, Faculty of Medicine, Institute of Microbiology and Immunology, Belgrade, Serbia.
- 1981 University Federico II of Naples, Department of Molecular Medicine and Medical Biotechnology, Naples, Italy.
- 1982 Universidade Federal do Rio de Janeiro, Institute of Biophysics Carlos Chagas Filho, Immunobiology Program, Rio de Janeiro, RJ, Brazil.
- 1983 National and Kapodistrian University of Athens, Faculty of Biology, Department of Cell Biology and Biophysics, Panepistimiopolis, Athens, Greece.
- 1984 Medical University of South Carolina, Department of Medicine, Division of Rheumatology & Immunology, Charleston, SC, USA.
- 1985 University of Bern, Institute of Pathology, Division of Experimental Pathology, Bern, Switzerland.
- 1986 University of Hong Kong, Cardiology Division, Department of Medicine, Hong Kong, China.
- 1987 University of Southampton , Biological Sciences, Faculty of Environmental and Life Sciences, Highfield, Southampton, UK.
- 1988 Universite de Moncton, Department of Chemistry and Biochemistry, Moncton, NB, Canada.
- 1989 University of Birmingham, Institute of Cancer and Genomic Sciences, Birmingham, UK.
- 1990 Weill Cornell Medicine, Department of Pathology and Laboratory Medicine, New York, NY, USA.
- 1991 Komarov Botanical Institute RAS, Laboratory of Molecular and Ecological Physiology, Saint Petersburg, Russian Federation.
- 1992 Nottingham Trent University, School of Science and Technology, Nottingham, UK.
- 1993 University of Oxford, John Radcliffe Hospital, Translational Gastroenterology Unit and Department of Paediatrics, Oxford, and Oxford NIHR Biomedical Research Centre, Oxford, UK.
- 1994 Institute of Psychiatry and Neurology, First Department of Neurology, Warsaw, Poland.
- 1995 Sechenov First Moscow State Medical University, Institute for Regenerative Medicine, Moscow, Russia.
- 1996 Mie University, Graduate School of Bioresources, Tsu, Japan.
- 1997 University of Osnabrück, Department of Biology/Chemistry, and Center of Cellular Nanoanalytics Osnabrück (CellNanOs), Osnabrück, Germany.
- 1998 Nagoya City University Graduate School of Medical Sciences, Department of Nephro-urology, Nagoya, Aichi, Japan.
- 1999 University of Tübingen, Center for Plant Molecular Biology (ZMBP), Tübingen, Germany.
- 2000 University of Milan, Department of Biosciences, Milan, Italy.
- 2001 University of Buenos Aires, Institute of Biochemistry and Molecular Medicine, Pathophysiology Department, School of Pharmacy and Biochemistry, CONICET, Buenos Aires, Argentina.
- 2002 University of Oxford, Nuffield Department of Clinical Neurosciences, Oxford, UK.
- 2003 Karolinska Institute, Department of Physiology and Pharmacology, Stockholm, Sweden.
- 2004 University of Murcia, Department of Biochemistry, Molecular Biology B and Immunology; Biomedical Research Institute of Murcia (IMIB-Arrixaca), Unit of Autophagy, Immunity and Cancer, Murcia, Spain.
- 2005 Fundación Instituto Leloir and IIBBA, CONICET, Buenos Aires, Argentina; Laboratorio de Glicobiología Celular y Genética Aplicada de Levaduras, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina.
- 2006 Instituto de Investigaciones Biomedicas Alberto Sols (CSIC-UAM), Madrid, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem), Instituto de Salud Carlos III, Madrid, Spain.
- 2007 Zhejiang University, School of Medicine, Department of Microbiology and Parasitology, Hangzhou, China.
- 2008 Vanderbilt University School of Medicine, Department of Pathology, Microbiology and Immunology, Nashville, TN, USA.
- 2009 Center of Translational Immunlogy, University Medical Center Utrecht, Utrecht, The Netherlands.
- 2010 Goethe University, Institute for Experimental Cancer Research in Pediatrics, Frankfurt am Main, Germany.
- 2011 University Hospitals Leuven, Department of Neurology, Leuven, Belgium; KU Leuven, Department of Neurosciences, Leuven, Belgium.
- 2012 Laboratory of Immunosenescence and Stem Cell Metabolism, Department of Oncology, Ludwig Cancer Institute, University of Lausanne, 1066 Epalinges, Switzerland.
- 2013 Scientific Institute, IRCCS E. Medea, Laboratory of Molecular Biology, Bosisio Parini, Lecco, Italy.
- 2014 Instituto de Investigaciones Biomedicas "Alberto Sols" (CSIC-UAM) and CIBERER (CIBER, ISCiii), Madrid, Spain.
- 2015 Harvard Medical School, Ophthalmology, Boston, MA, USA.
- 2016 Universitätsklinikum Essen, Institute for Cell Biology (Cancer Research), Essen, Germany.
- 2017 University of Groningen, University Medical Center Groningen, Department of Hematology, Groningen, The Netherlands.
- 2018 Tuscia University, Department of Ecological and Biological Sciences (DEB), Viterbo, Italy.
- 2019 EA 3842, Limoges University, Faculty of Medicine, Limoges cedex, France.
- 2020 Biomedical Research Foundation, Academy of Athens, Center of Clinical, Experimental Surgery and Translational Research, Athens, Greece.
- 2021 Institut de Pharmacologie et de Biologie Structurale, Université de Toulouse, CNRS, Université Paul Sabatier, Toulouse, France.
- 2022 University of Pittsburgh, Department of Pathology, Pittsburgh, PA, USA.
- 2023 Washington University in St. Louis, Department of Medicine, St. Louis, MO, USA.
- 2024 Service of Endocrinology, University Hospital Doctor Peset, Foundation for the Promotion of Health and Biomedical Research in the Valencian Region (FISABIO)-Department of Physiology, University of Valencia, Valencia, Spain.
- 2025 University of Montpellier, UMR 5235, Montpellier, France.
- 2026 Universidade NOVA de Lisboa, CEDOC, NOVA Medical School, Faculdade de Ciências Médicas, Lisboa, Portugal.
- 2027 University of Helsinki, Institute of Biotechnology, Electron Microscopy Unit, Helsinki, Finland.
- 2028 Vall d'Hebron Research Institute (VHIR)-Network Center for Biomedical Research in Neurodegenerative Diseases (CIBERNED)-Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain.
- 2029 Institute of Biomedicine of Valencia, Spanish Research Council (CSIC), Valencia, Spain.
- 2030 University Hospital La Paz Research Institute (IdiPAZ), Cancer and Human Molecular Genetics Area Oto-Neurosurgery Research Group, Madrid, Spain.
- 2031 University of Toulouse, Institute of Metabolic and cardiovascular Diseases, INSERM UMR 1048, Toulouse, France.
- 2032 Università Cattolica del Sacro Cuore, Department of Life Science and Public Health Section of Histology and Embryology, Rome, Italy.
- 2033 Italian Institute for Genomic Medicine (IIGM), Candiolo (TO), Italy; Candiolo Cancer Institute, FPO-IRCCS, Candiolo (TO), Italy.
- 2034 Simon Fraser University, Department of Chemistry, Department of Molecular Biology and Biochemistry, Burnaby, British Columbia, Canada.
- 2035 Institute for Systems Analysis and Computer Science "A. Ruberti", National Research Council (IASI-CNR), IRCCS Fondazione Santa Lucia, Rome, Italy.
- 2036 Eurac Research, Institute for Biomedicine, Bolzano, Italy.
- 2037 Rheinische Friedrich-Wilhelms-Universität, Institut für Biochemie und Molekularbiologie (IBMB), Bonn, Germany.
- 2038 University of Oxford, Oxford Parkinson's Disease Centre, Department of Physiology, Anatomy and Genetics, Oxford, UK.
- 2039 Fukushima Medical University, School of Medicine, Department of Anatomy and Histology, Fukushima, Japan.
- 2040 University of California, Los Angeles, Department of Integrative Biology and Physiology, Los Angeles, CA, USA.
- 2041 The University of Queensland, School of Chemistry and Molecular Biosciences and Australian Infectious Diseases Research Centre, St. Lucia, Brisbane, Queensland, Australia.
- 2042 Babraham Institute, Cambridge, UK.
- 2043 University of Bonn, Department of Neurology, Molecular Cell Biology Unit, Bonn, Germany.
- 2044 Universidad Autónoma de Madrid, Centro de Biología Molecular Severo Ochoa (CSIC-UAM & CIBERNED), Madrid, Spain.
- 2045 St Jude Children's Research Hospital, Department of Pathology, Memphis, TN, USA.
- 2046 Chang Gung University College of Medicine and Chang Gung Memorial Hospital, Department of Cardiology, Taoyuan City, Taiwan; National Health Research Institutes, Institute of Cellular and System Medicine, Zhunan, Taiwan.
- 2047 China Pharmaceutical University, School of Life Science and Technology, Nanjing, Jiangsu, China.
- 2048 University of California Los Angeles, School of Dentistry, Division of Oral Biology and Medicine, Los Angeles, CA, USA.
- 2049 Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China.
- 2050 University of Michigan, Department of Internal Medicine, Division of Hematology and Oncology, Ann Arbor, MI, USA.
- 2051 Shanghai Ninth People's Hospital, National Clinical Research Center for Oral Disease, Shanghai Jiao Tong Univeristy, School of Medicine, Shanghai, China.
- 2052 Texas A&M College of Dentistry, Department of Endodontics, Dallas, TX, USA.
- 2053 Third Military Medical University of China, Xinqiao Hospital, Institute of Respiratory Diseases, Chongqing, China.
- 2054 Soochow University, Center for Circadian Clocks; School of Biology & Basic Medical Sciences, Medical College, Suzhou, Jiangsu, China.
- 2055 CAS Center for Excellence in Nanoscience, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology (NCNST), Zhongguancun, Beijing, China.
- 2056 China Agricultural University, College of Biological Sciences, State Key Laboratory of Agro-Biotechnology and MOA Key Laboratory of Soil Microbiology, Beijing, China.
- 2057 Soochow University, Hematology Center of Cyrus Tang Hematology Institute, School of Medicine, Suzhou, China.
- 2058 China Academy of Chinese Medical Sciences, Artemisinin Research Center and the Institute of Chinese Materia Medica, Beijing, China.
- 2059 Shanxi Agricultural University, Shanxi Key Laboratory of Ecological Animal Science and Environmental Veterinary Medicine, Taigu, Shanxi, China.
- 2060 Sichuan University, West China School of Basic Medical Sciences & Forensic Medicine, Chengdu, China.
- 2061 Hunan Key Laboratory of Medical Epigenomics, Department of Dermatology, Second Xiangya Hospital, Central South University, Changsha, China.
- 2062 The Chinese University of Hong Kong, School of Public Health and Primary Care, Hong Kong, China.
- 2063 Fourth Military Medical University, College of Stomatology, Department of Oral Anatomy and Physiology and TMD, Xi'an, China.
- 2064 The First Affiliated Hospital, Jinan University, Guangzhou, China.
- 2065 Huazhong Agricultural University, College of Horticulture and Forestry Science, Key Laboratory of Horticultural Plant Biology (Ministry of Education), Wuhan, China.
- 2066 National University of Singapore, Department of Medicine, Cardiovascular Research Institute, Singapore.
- 2067 Feinstein Institutes for Medical Research, Center for Immunology and Inflammation, Manhasset, NY, USA.
- 2068 University of Kentucky, Department of Ophthalmology and Visual Sciences, Lexington, KY, USA.
- 2069 Zhujiang Hospital of Southern Medical University, Department of Neurology, Guangzhou, Guangdong Province, China.
- 2070 Cleveland Clinic, Department of Cardiovascular Medicine/Heart and Vascular Institute, Cleveland, OH, USA.
- 2071 Diabetes and Metabolism Research Institute, Department of Molecular & Cellular Endocrinology; City of Hope Medical Center, Beckman Research Institute, Comprehensive Cancer Center, Duarte, CA, USA.
- 2072 Xuzhou Medical University, Department of Anesthesiology, Xuzhou, Jiangsu, China.
- 2073 Stanford University School of Medicine, Department of Neurosurgery, Palo Alto, CA, USA.
- 2074 University of South Dakota, Division of Basic Biomedical Sciences, Vermillion, SD, USA.
- 2075 Department of Biomedical Sciences, College of Medicine, Florida State University, Tallahassee, FL, USA.
- 2076 Department of Neurology, University of Michigan School of Medicine, Ann Arbor, MI, USA.
- 2077 Kaohsiung Medical University, School of Dentistry, College of Dental Medicine, Kaohsiung, Taiwan.
- 2078 University of Southampton, Faculty of Environmental and Life Sciences, Biological Sciences, and the Institute for Life Sciences, Southampton, UK.
- 2079 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany; Max-Planck-Institute for Metabolism Research, Hamburg Outstation, Hamburg, Germany; Shanghai Qiangrui Biotech Co., Ltd., Fengxian District, Shanghai, China.
- 2080 Nanjing Agricultural University, College of Horticulture, Weigang NO. 1, Nanjing, China.
- 2081 Saint Louis University, Department of Biology, Saint Louis, MO, USA.
- 2082 Air Force Medical University, Xijing Hospital and School of Basic Medicine, Department of Pathology, State Key Laboratory of Cancer Biology, Xi'an, China.
- 2083 Affiliated Union Hospital of Fujian Medical University, Fuzhou, Fujian, China.
- 2084 Wenzhou Medical University, Key Laboratory of Biotechnology and Pharmaceutical Engineering, School of Pharmaceutical Sciences, Wenzhou, China.
- 2085 Queen Mary University, Blizard Institute, Flow Cytometry Core Facility, London, UK.
- 2086 Juntendo University Graduate School of Medicine, Department of Metabolism & Endocrinology, Bunkyo-ku, Tokyo, Japan.
- 2087 Chiba University Graduate School of Medicine, Department of General Medical Science, Chiba, Japan.
- 2088 University of Cincinnati College of Medicine, Cincinnati Children's Research Foundation, Division of Pulmonary Biology, Department of Pediatrics, Cincinnati, OH, USA.
- 2089 Department of Biological Sciences, University of Denver, Denver, CO, USA.
- 2090 University of Pennsylvania, Department of Anesthesiology, Philadelphia, PA, USA.
- 2091 Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China.
- 2092 Washington University School of Medicine, Department of Neurology, St. Louis, MO, USA.
- 2093 University of Bonn, Pharmaceutical Institute, Section Pharmacology and Toxicology, Bonn, Germany.
- 2094 University Hospital RWTH Aachen, Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC), Aachen, Germany.
- 2095 Univ of Pittsburgh and Pittsburgh VA HealthSystem, Department of Pathology, Pittsburgh, PA, USA.
- 2096 Spencer Center for Vision Research, Byers Eye Institute, Stanford University School of Medicine, Stanford, CA, USA.
- 2097 Georg August University Göttingen, Institute of Microbiology and Genetics, Department of Genetics of Eukaryotic Microorganisms, Göttingen, Germany.
- 2098 Harvard Medical School and Brigham & Women's Hospital, Precision Neurology Program & APDA Center for Advanced Parkinson Research, Boston, MA, USA.
- 2099 University of Nottingham, School of Life Sciences, Queen's Medical Centre, Nottingham, UK.
- 2100 Scripps Research Institute, La Jolla, CA, USA.
- 2101 Amsterdam University Medical Centers, Location AMC, Tytgat Institute for Intestinal Research, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands.
- 2102 Quadram Institute Bioscience, Norwich Research Park, Norwich, Norfolk, UK.
- 2103 University of Edinburgh, MRC Institute of Genetics and Molecular Medicine, Scotland, UK.
- 2104 Heinrich-Heine-Universität Düsseldorf Institut für Physikalische Biologie, Düsseldorf, Germany.
- 2105 Queensland University of Technology, Centre for Tropical Crops and Biocommodities, Science and Engineering Faculty, Brisbane, Queensland, Australia.
- 2106 Royal Holloway University of London, Centre for Biomedical Sciences, Egham, Surrey, UK.
- 2107 National Institutes of Health, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
- 2108 University of Nebraska-Lincoln, Department of Plant Pathology, Lincoln, NE, USA.
- 2109 Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Department of Stem Cell Biology, Erlangen, Germany.
- 2110 University of Toronto, Faculty of Medicine, Department of Immunology, Toronto, ON, Canada.
- 2111 Department of Obstetrics and Gynecology, Weill Cornell Medicine, 1300 York Avenue, Box 35, New York, NY, United States.
- 2112 MFP CNRS UMR 5234, University of Bordeaux, Bordeaux, France.
- 2113 Institut Pasteur, Membrane Biochemistry and Transport, Paris, France.
- 2114 National University of Singapore, Yong Loo Lin School of Medicine, Healthy Longevity Translational Research Program, Singapore; National University Health System, Centre for Healthy Longevity, Singapore.
- 2115 School of Medicine, Shenzhen University, Shenzhen, China.
- 2116 Kanazawa University, WPI-Nano Life Science Institute (WPI-nanoLSI), Kanazawa, Ishikawa, Japan.
- 2117 University of Zurich, Institute of Experimental Immunology, Switzerland, Zurich.
- 2118 Southwest Medical University, Luzhou Key Laboratory of Activity Screening and Druggability Evaluation for Chinese Materia Medica, Jiangyang District, Luzhou, China.
- 2119 University of California San Diego, Department of Neurosciences, La Jolla, CA, USA.
- 2120 University of Maryland School of Medicine, Center for Shock, Trauma and Anesthesiology Research (STAR), Departments of Anesthesiology, Anatomy & Neurobiology, Baltimore, MD, USA.
- 2121 NHRI, Institute of Cellular and System Medicine, Zhunan, Taiwan.
- 2122 University of North Dakota, Biology School of Medicine and Health Sciences, Department of Biomedical Sciences, Grand Forks, ND, USA.
- 2123 Soochow University, Department of Pathogenic Biology, Suzhou, Jiangsu Province, China.
- 2124 First Affiliated Hospital of Xi'an Jiaotong University School of Medicine, Center for Translational Medicine, Xi'an, Shaanxi, China.
- 2125 Zhejiang Provincial People's Hospital, Department of Endocrinology, Hangzhou, China.
- 2126 University of Kansas, Department of Molecular Biosciences, and University of Kansas Medical School, University of Kansas Cancer Center, Department of Radiation Oncology, Lawrence, KS, USA.
- 2127 Zhejiang University School of Medicine, Department of Toxicology of School of Public Health, and Department of Gynecologic Oncology of Women's Hospital, Hangzhou,China.
- 2128 The Broad Institute of Harvard and MIT, Massachusetts General Hospital, Harvard Medical School, Department of Molecular Biology, Cambridge, MA, USA.
- 2129 Zhejiang University, Department of Biochemistry in School of Medicine, Hangzhou, Zhejiang Province, China.
- 2130 Shenzhen University, College of Medicine, Shenzhen, Guangdong, China.
- 2131 The University of Hong Kong, Department of Anesthesiology, Queen Mary Hospital, Hong Kong, China.
- 2132 Wuhan University, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education, School of Pharmaceutical Sciences, Wuhan, China.
- 2133 Shanghai Jiao Tong University School of Medicine, Ruijin Hospital, Department of Otolaryngology & Head and Neck Surgery, Shanghai, China.
- 2134 Friedrich-Alexander University Erlangen-Nürnberg, University Hospital Erlangen, Department of Molecular Neurology, Erlangen Germany.
- 2135 Research Department, National Neuroscience Institute, Singapore.
- 2136 Southern University of Science and Technology, Department of Biology, Shenzhen, China.
- 2137 Sichuan University, West China Hospital, Centre of Geriatrics and Gerontology, Chengdu, China.
- 2138 University of Electronic Science and Technology of China, School of Medicine, Chengdu, China; Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, Department of Pharmacy, Chengdu, Sichuan, China.
- 2139 Wenzhou Medical University, School of Pharmaceutical Sciences, Wenzhou, China.
- 2140 Queensland University of Technology, Institute of Health and Biomedical Innovation, Kelvin Grove, Brisbane, Queensland, Australia.
- 2141 Sun Yat-sen University, School of Life Sciences, Guangdong Provincial Key Laboratory of Plant Resources, State Key Laboratory of Biocontrol, Guangzhou, China.
- 2142 South China Agricultural University, College of Veterinary Medicine, Guangzhou, Guangdong, China.
- 2143 Southwest Hospital, Third Military Medical University (Army Medical University), Key Laboratory of Hepatobiliary and Pancreatic Surgery, Institute of Hepatobiliary Surgery, Chongqing, China.
- 2144 University of Alabama at Birmingham, Division of Cardiovascular Disease, Department of Medicine, Birmingham, AL, USA.
- 2145 Georgia State University, The Center for Molecular and Translation Medicine, Atlanta, GA, USA.
- 2146 Biomedical Research Foundation of the Academy of Athens (BRFAA), Center of Clinical, Experimental Surgery, & Translational Research, Athens, Greece.
- 2147 Shanghai Sixth People's Hospital, Department of Cardiovascular Medicine, and Shanghai Jiaotong University School of Medicine, Shanghai Institute of Immunology, Shanghai, China.
- 2148 Institute of Neurosciences and Department of Neurology of the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- 2149 Jinan University, College of Life Science and Technology, Guangzhou, China.
- 2150 Yangzhou University, College of Veterinary Medicine, Jiangsu Province, China.
- 2151 Kyoto Institute of Technology, Department of Applied Biology, Matsugasaki, Sakyo-ku, Kyoto, Japan.
- 2152 Ehime University Graduate School of Medicine, Department of Cardiology, Pulmonology, Hypertension & Nephrology, Shitsukawa, Toon, Ehime, Japan.
- 2153 Columbia University, Department of Neurology, and Pathology and Cell Biology, New York, NY, USA.
- 2154 Juntendo University School of Medicine, Department of Gastroenterology, Bunkyo-ku, Tokyo, Japan.
- 2155 Indiana University School of Medicine, Department of Pathology and Laboratory Medicine, Indianapolis, IN, USA.
- 2156 National Cheng Kung University, College of Medicine, Department of Physiology, Tainan City, Taiwan.
- 2157 University of Virginia, Departments of Medicine, Pharmacology, Molecular Physiology & Biological Physics, Center for Skeletal Muscle Research at Robert M. Berne Cardiovascular Research Center, University of Virginia School of Medicine, Charlottesville, VA, USA.
- 2158 Asahikawa Medical University, Department of Ophthalmology, Hokkaido, Japan.
- 2159 Hong Kong Baptist University, Mr. and Mrs. Ko Chi Ming Centre for Parkinson's Disease Research, School of Chinese Medicine, Hong Kong, China.
- 2160 Nathan Kline Institute for Psychiatric Research, Center for Dementia Research, Orangeburg, NY; New York University Langone Health, Department of Psychiatry, New York, NY, USA.
- 2161 University of Wisconsin-Madison, Department of Surgery, Madison, WI, USA.
- 2162 Chinese Academy of Sciences, Shanghai Institute of Nutrition and Health, CAS Key Laboratory of Tissue Microenvironment & Tumor, Laboratory of Molecular Cardiology, Shanghai, China.
- 2163 Capital Medical University, School of Basic Medical Sceinces, Department of Neurobiology, Beijing, China.
- 2164 University of Kentucky, Department of Cancer Biology & Toxicology, Lexington, KY, USA.
- 2165 University of Alabama at Birmingham, Department of Medicine, Division of Cardiovascular Disease, Birmingham, AL, USA.
- 2166 University of Iowa Carver College of Medicine, Fraternal Order of Eagles Diabetes Research Center, Department of Anatomy and Cell Biology, Iowa City, IA, USA.
- 2167 The Fourth Military Medical University, Xijing Hospital, Department of Orthopaedics, Xi'an, China.
- 2168 Shandong Cancer Hospital and Institute, Cancer Research Center, Jinan, Shandong Province, China.
- 2169 Taipei Medical University, College of Medical Science and Technology, Graduate Institute of Cancer Biology and Drug Discovery, Taipei, Taiwan.
- 2170 The Fourth Military Medical University, Tangdu Hospital, Department of Experimental Surgery, Xi'an, Shaanxi, China.
- 2171 Chung Shan Medical University, Institute of Medicine, Taichung, Taiwan.
- 2172 Jiangsu University, School of Medicine, Zhenjiang, Jiangsu, China.
- 2173 Yale University School of Medicine, Department of Comparative Medicine and Department of Cellular and Molecular Physiology, New Haven, CT, USA.
- 2174 Jinan University, Medical College, Key Laboratory for Regenerative Medicine of the Ministry of Education, Division of Histology and Embryology, Guangzhou, China.
- 2175 Hangzhou Normal University, School of Medicine, College of Pharmacy, Hangzhou, Zhejiang, China.
- 2176 University of Southeast, School of Medicine, Department of Pharmacology, Nanjing, JiangSu, China.
- 2177 University of Melbourne, Bio21 Molecular Science and Biotechnology Institute, Parkville, Victoria, Australia.
- 2178 Chinese University of Hong Kong, School of Biomedical Sciences, Hong Kong, China.
- 2179 Fourth Medical Center of the Chinese PLA General Hospital, Trauma Research Center, Beijing, China.
- 2180 Duke-NUS Medical School, Cardiovascular and Metabolic Disorders Program, Singapore, Singapore.
- 2181 Zhejiang University School of Medicine, Department of Biochemistry, and Department of Hepatobiliary and Pancreatic Surgery of the First Affiliated Hospital, Hangzhou, China.
- 2182 Shandong Agricultural University, College of Plant Protection, Shandong, China.
- 2183 Zhejiang University, College of Pharmaceutical Sciences, Hangzhou, China.
- 2184 Soochow University, College of Pharmaceutical Sciences, Department of Pharmacology, Suzhou, Jiangsu, China.
- 2185 The University of British Columbia, Department of Biochemistry and Molecular Biology, Vancouver, BC, Canada.
- 2186 Dong-A University College of Medicine, Department of Anatomy and Cell Biology, Busan, Korea.
- 2187 The Jikei University School of Medicine, Department of Biochemistry, Minato-ku, Tokyo, Japan.
- 2188 ETH Zürich, Institute of Biochemistry, Zürich, Switzerland.
- 2189 Tabriz University of Medical Sciences, Molecular Medicine Research Center, Tabriz, Iran.
- 2190 Hwa Chong Institution, Singapore, Singapore.
- 2191 Tianjin University of Traditional Chinese Medicine, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin, China.
- 2192 Temple University, Center for Metabolic Disease Research and Department of Physiology, Philadelphia, PA, USA.
- 2193 The Chinese University of Hong Kong, State Key Laboratory of Digestive Disease, Department of Medicine and Therapeutics, Hong Kong, China.
- 2194 Tsinghua University, School of Life Sciences, Tsinghua University-Peking University Joint Center for Life Sciences, State Key Laboratory of Membrane Biology, Beijing, China.
- 2195 Hepatobiliary Section, Department of Internal Medicine, Kaohsiung Medical University Hospital, and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 2196 University of Toronto, Centre for Addiction and Mental Health, Toronto, Ontario, Canada.
- 2197 Air Force Medical University, Xijing Hospital, Department of Plastic and Reconstructive Surgery, Xi'an, China.
- 2198 Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 201203 Shanghai, China; Department of Cell Biology, Harvard Medical School, Boston, MA, USA.
- 2199 Central South University, The Second Xiang-Ya Hospital, National Clinical Research Center for Metabolic Diseases, Department of Metabolism and Endocrinology, Changsha, Hunan, China.
- 2200 Kaohsiung Medical University, Graduate Institute of Medicine, Kaohsiung, Taiwan.
- 2201 First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China.
- 2202 Beijing Institute of Basic Medical Sciences, Beijing, China.
- 2203 City University of Hong Kong, Department of Biomedical Sciences, Hong Kong, China.
- 2204 Icahn School of Medicine at Mount Sinai, Departments of Neurology and Neuroscience, New York, NY, USA.
- 2205 Graduate School of Dong-A University, College of Medicine, Department of Translational Biomedical Sciences, Busan, Korea.
- 2206 Centre for Genomic Regulation (CRG), Department of Cell and Developmental Biology, Barcelona, España.
- 2207 Laboratory of Pain and Signaling, Butantan Institute, Sao Paulo, SP, Brazil; Stanford University, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford, CA, USA.
- 2208 University of Brescia, Department of Molecular and Translational Medicine, Brescia, Italy.
- 2209 University of Texas Southwestern Medical Center, Department of Surgery, Dallas, TX, USA.
- 2210 Cancer Research UK Beatson Institute, Glasgow, UK; University of Glasgow, Institute of Cancer Sciences, Glasgow, UK.
- 2211 University of California, Davis, Department of Pathology and Laboratory Medicine, Shriners Hospitals for Children, Institute for Pediatric Regenerative Medicine, Sacramento, CA, USA.
- 2212 Tabriz University of Medical Sciences, Faculty of Advanced Medical Sciences, Department of Medical Nanotechnology, Tabriz, Iran.
- 2213 University Monastir, Faculty of Medicine, LR12ES05, Lab-NAFS 'Nutrition - Functional Food & Vascular Health', Monastir, Tunisia.
- 2214 University of Virginia, Department of Neuroscience, Charlottesville, VA, USA.
- 2215 Boston University, Department of Biomedical Engineering, Boston, MA, USA.
- 2216 University of Arizona, Department of Pharmacology and Toxicology, Tucson, AZ, USA.
- 2217 Peking University First Hospital, Renal Division, Xi Cheng District, Beijing, China.
- 2218 Chinese Academy of Sciences, Institute of Biophysics, National Laboratory of Biomacromolecules, Beijing, China.
- 2219 Zhejiang University School of Medicine, Department of Pathology, Hangzhou, China.
- 2220 University of Science & Technology of China, School of Life Sciences, Hefei, Anhui, China.
- 2221 Rutgers Robert Wood Johnson Medical School, Department of Neuroscience and Cell Biology, Piscataway, NJ, USA.
- 2222 Thomas Jefferson University/Vickie & Jack Farber Institute for Neuroscience, Hospital for Neuroscience, Philadelphia, PA, USA.
- 2223 Soochow University, Department of Pharmacology and Laboratory of Cerebrovascular Pharmacology, College of Pharmaceutical Science, Suzhou, Jiangsu, China.
- 2224 People's Hospital of Hangzhou Medical College, Zhejiang Provincial People's Hospital, Department of Oncology, Hangzhou, China; Zhejiang Provincial People's Hospital, Clinical Research Institute, Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
- 2225 University of Alabama at Birmingham, Department of Pathology, Birmingham, AL, USA.
- 2226 School of Radiation Medicine and Protection and State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, China.
- 2227 The Chinese University of Hong Kong, Department of Medicine and Therapeutics and Department of Anaesthesia and Intensive Care, Hong Kong, China.
- 2228 Huazhong Agricultural University, School of Veterinary Medicine/ Biomedical Center, Hongshan, Wuhan, China.
- 2229 Henan University of Technology, College of Bioengineering, Zhengzhou, China.
- 2230 University of Utah, Huntsman Cancer Institute and Department of Oncological Sciences, Salt Lake City, UT, USA.
- 2231 The University of Texas Health Science Center at Houston (UTHealth), McGovern Medical School, The Brown Foundation Institute of Molecular Medicine, Department of Neurobiology and Anatomy, Programs in Human and Molecular Genetics and Neuroscience of the MD Anderson UTHealth GSBS, Houston, TX, USA.
- 2232 Wake Forest Baptist Comprehensive Cancer Center, Department of Cancer Biology, Winston-Salem, NC, USA.
- 2233 Hungarian Academy of Sciences, Budapest, Hungary; Department of Anatomy, Cell and Developmental Biology, Eötvös Loránd University, Budapest, Hungary.
- 2234 The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Department of Orthopaedics, Wenzhou, Zhejiang Province, China.
- 2235 College of Respiratory and Critical Care Medicine, Chinese PLA General Hospital, Beijing, China.
- 2236 University of Newcastle, School of Biomedical Sciences and Pharmacy, NSW, Australia.
- 2237 University of Houston, College of Pharmacy, Department of Pharmacological & Pharmaceutical Sciences, Houston, TX, USA.
- 2238 University of Maryland, Molecular Virology Laboratory, VA-MD College of Veterinary Medicine, College Park, MD, USA.
- 2239 East China Normal University, Key Laboratory of Adolescent Health Assessment and Exercise Intervention of Ministry of Education, Shanghai, China.
- 2240 Nanjing Agricultural University, College of Plant Protection, Department of Plant Pathology, Nanjing, China.
- 2241 Department of Physiology, Department of Obstetrics/Gynecology, Wayne State University, Detroit, MI, USA.
- 2242 Rutgers University, Department of Surgery, New Brunswick, NJ, USA.
- 2243 Nanjing University of Chinese Medicine, Nanjing, China.
- 2244 Central China Normal University, College of Life Sciences, Wuhan, Hubei Province, China.
- 2245 The Fourth Military Medical University, Tangdu Hospital, Department of Neurosurgery, Xi'an, Shaanxi, China.
- 2246 Beijing Academy of Agriculture and Forestry Sciences, Institute of Plant and Environment Protection, Beijing, China.
- 2247 Shandong University, School of Life Sciences, Shandong Provincial Key Laboratory of Animal Cells and Developmental Biology, Qingdao, China.
- 2248 Peking University Health Sciences Center, Department of Biochemistry and Molecular Biology, Beijing, China.
- 2249 Zhejiang University, the First Affiliated Hospital, Hangzhou, Zhejiang, China.
- 2250 Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China.
- 2251 University of Sydney, Westmead Institute for Medical Research, Centre for Transplant and Renal Research, Sydney NSW, Australia.
- 2252 Shenzhen University, Health Science Center, School of Pharmaceutical Sciences, Shenzhen, Guangdong, China.
- 2253 Wuhan University, Department of Cell Biology, College of Life Sciences, Wuhan, Hubei, China.
- 2254 University of Calgary, Cumming School of Medicine, Libin Cardiovascular Institute of Alberta, Departments of Biochemistry & Molecular Biology and Physiology & Pharmacology, Calgary, Alberta, Canada.
- 2255 Children's Hospital Medical Center, Division of Experimental Hematology and Cancer Biology, Cincinnati, OH, USA.
- 2256 China Pharmaceutical University, State Key Laboratory of Natural Medicines, Nanjing, Jiangsu, China.
- 2257 Karolinska Institutet Division of Toxicology, Institute of Environmental Medicine, Stockholm, Sweden; Lomonosov Moscow State University, Faculty of Basic Medicine, Moscow, Russia.
- 2258 Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Department of Pathophysiology, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai, China.
- 2259 Southwest Medical University, Department of Medical Cellular Biology, Luzhou, Sichuan, China.
- 2260 Massachusetts General Hospital, Department of Medicine, Diabetes Unit and Center for Genomic Medicine, Boston, MA, USA; Harvard Medical School, Department of Medicine, Boston, MA, USA.
- 2261 Chinese People's Liberation Army General Hospital, Department of Cardiology, Beijing, China.
- 2262 Third Military Medical University, College of Pharmacy, Department of Pharmacology, Chongqing, China.
- 2263 Huazhong Agricultural University, College of Veterinary Medicine, State Key Laboratory of Agricultural Microbiology, Wuhan, China.
- 2264 Zhejiang University, Department of Horticulture, Hangzhou, China.
- 2265 Guangxi Medical University, School of Preclinical Medicine, Department of Physiology, Nanning,China.
- 2266 Zhejiang University, MOA Key Laboratory of Animal Virology, Hangzhou, Zhejiang, China.
- 2267 The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Department of Orthopaedics, Wenzhou, Zhejiang, China.
- 2268 Chinese Academy of Sciences, Guangzhou Institutes of Biomedicine and Health, CAS Key Laboratory of Regenerative Biology, Guangzhou, China.
- 2269 Queensland University of Technology, Institute of Health and Biomedical Innovation, Brisbane, QLD, Australia.
- 2270 Texas A&M University, Institute of Biosciences and Technology, Houston, TX, USA.
- 2271 Soochow University, Laboratory Animal Center, Suzhou, Jiangsu Province, China.
- 2272 Zhejiang University School of Medicine, Department of Environmental Medicine, Hangzhou, Zhejiang Province, China.
- 2273 University at Buffalo, The State University of New York, Department of Physiology and Biophysics, Buffalo, NY, USA.
- 2274 Zhengzhou University, Third Affiliated Hospital and Institute of Neuroscience, Henan Key Laboratory of Child Brain Injury, Zhengzhou, China; Gothenburg University, Institute of Neuroscience and Physiology, Gothenburg, Sweden.
- 2275 Nanjing Medical University, Department of Physiology, Nanjing, Jiangsu, China.
- 2276 University of Kentucky, College of Medicine, Department of Molecular and Cellular Biochemistry, Lexington, KY, USA.
- 2277 Shanghai Jiao Tong University, Bio-X Institutes, Shanghai, China.
- 2278 The Ohio State University, Department of Surgery, Columbus, OH, USA.
- 2279 Shenzhen University Medical School, Department of Biochemistry and Molecular Biology, Shenzhen, China.
- 2280 State Key Laboratory of Medicinal Chemical Biology, Tianjin Key Laboratory of Protein Science, College of Life Sciences, Nankai University, Tianjin, China.
- 2281 University California, San Diego, Division of Biological Sciences, Section of Molecular Biology, CA, USA.
- 2282 University Medical Center of Johannes Gutenberg-University, Institute for Virology, Mainz, Germany.
- 2283 University of Padova, Biology Department, Padova, Italy.
- 2284 Rutgers University, Department of Chemical Biology, Ernest Mario School of Pharmacy, Piscataway, NJ, USA.
- 2285 Moscow State University, A.N.Belozersky Institute of Physico-Chemical Biology, Department of Functional Biochemistry of Biopolymers, Moscow, Russia.
- 2286 University of Michigan, Departments of Surgery and Pathology, Ann Arbor, MI, USA.
- 2287 Institute of Life Sciences, Chongqing Medical University, Chongqing, PR China.
- 2288 The University of Queensland, Queensland Brain Institute, Clem Jones Centre for Ageing Dementia Research, Brisbane, Australia.
- 2289 University of Innsbruck, Division of Cell Metabolism and Differentiation Research, Research Institute for Biomedical Aging Research, Innsbruck, Austria.
- 2290 Indiana University School of Medicine, Department of Biochemistry and Molecular Biology, Indianapolis, IN, USA.
- 2291 Swedish University of Agricultural Sciences, Department of Forest Mycology and Plant Pathology, Uppsala, Sweden.
- 2292 Southern Medical University, Shenzhen Hospital, Department of Pathology, Bao'an District, Shenzhen, Guangdong, China.
- 2293 Lovelace Respiratory Research Institute, Molecular Biology and Lung Cancer Program, Albuquerque, NM, USA.
- 2294 Tokyo Medical and Dental University (TMDU), Medical Hospital, Department of Gastroenterology and Hepatology, Bunkyo-ku, Tokyo, Japan.
- 2295 Huazhong University of Science and Technology, School of Basic Medicine, Wuhan, HuBei, China.
- 2296 Maastricht University Medical Center, School for Cardiovascular disease (CARIM), Department of Pathology, Maastricht, The Netherlands; University of Edinburgh, Centre for Cardiovascular Science, Edinburgh, UK.
- 2297 Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO, USA.
- 2298 Hong Kong Baptist University, School of Chinese Medicine, Hong Kong, China.
- 2299 Hong Kong Baptist University, School of Chinese Medicine, Hong Kong, lxChina.
- PMID: 33634751
- PMCID: PMC7996087
- DOI: 10.1080/15548627.2020.1797280
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1
Authors
Affiliations
- 1 Life Sciences Institute and Department of Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, MI, USA.
- 2 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt; Department of Experimental Oncology, IEO, European Institute of Oncology IRCCS, Milan, Italy.
- 3 Johannes Gutenberg University, Institute of Pharmaceutical and Biomedical Sciences, Department of Pharmaceutical Biology, Mainz, Germany.
- 4 Medical University of Graz, Division of Cardiology, Graz, Austria.
- 5 Pasteur institute of Iran, Department of Hepatitis and HIV, Tehran, Iran.
- 6 Department of Molecular Signal Processing, Leibniz Institute of Plant Biochemistry, Halle (Saale), Germany.
- 7 Hebrew University of Jerusalem, Department of Biochemistry and Food Science, Rehovot, Israel.
- 8 Danish Cancer Society Research Center, RNA and Autophagy Group, Copenhagen, Denmark.
- 9 University of Copenhagen, Biotech Research and Innovation Centre, Copenhagen, Denmark.
- 10 University of Tromsø - The Arctic University of Norway, Department of Medical Biology, Tromsø, Norway.
- 11 Hospital Universitario de Canarias, Research Unit; CIBERNED and Universidad de La Laguna, ITB, Santa Cruz de Tenerife, Spain.
- 12 University of California, Davis, Division of Rheumatology, Allergy and Clinical Immunology, School of Medicine, Sacramento, CA, USA.
- 13 University of Toronto, Department of Biochemistry, Toronto, Ontario, Canada.
- 14 Medical University Innsbruck, Department of Medicine I, Innsbruck, Austria.
- 15 University of Calabria, Department of Pharmacy, Health and Nutritional Sciences, Arcavacata di Rende (CS), Italy.
- 16 National Institutes of Health, National Eye institute, Protein Structure and Function Section, Bethesda, MD, USA.
- 17 Ben-Gurion University of the Negev, Faculty of Health Sciences, Department of Clinical Biochemistry and Pharmacology, Beer-Sheva, Israel.
- 18 University of Alabama at Birmingham, Division of Nephrology and Birmingham VA Medical Center, Birmingham, AL, USA.
- 19 Inflammation Research Center, San Diego, CA, USA.
- 20 University of Palermo, Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche (STEBICEF), Palermo, Italy.
- 21 Cell Death Research & Therapy (CDRT) Lab, Department of Cellular & Molecular Medicine, Center for Cancer Biology, VIB-KU Leuven, Leuven, Belgium.
- 22 Indian Institute of Technology, Ropar, India.
- 23 University of Dundee, School of Life Sciences, MRC Protein Phosphorylation and Ubiquitylation Unit, Dundee, UK.
- 24 University of Texas Medical Branch, Department of Pathology, Galveston, TX, USA.
- 25 Colby College, Department of Biology, Waterville, ME, USA.
- 26 University of Maryland School of Medicine, Departments of Otorhinolaryngology Head & Neck Surgery, Ophthalmology and Visual Science, Baltimore, MD, USA.
- 27 Universidad Mayor, Center for Integrative Biology, and Geroscience Center for Brain Health and Metabolism, Santiago, Chile.
- 28 Lund University, Department of Experimental Medical Science, Cell Death, Lysosomes and Artificial Intelligence Group, Lund, Sweden.
- 29 Nihon University, College of Bioresource Sciences, Fujisawa, Kanagawa, Japan.
- 30 Koç University School of Medicine, Department of Molecular Biology and Genetics and KUTTAM Research Center for Translational Medicine, Istanbul, Turkey.
- 31 University of Crete, School of Medicine, Laboratory of Clinical Microbiology and Microbial Pathogenesis, Voutes, Heraklion, Crete, Greece; Foundation for Research and Technology, Institute of Molecular Biology and Biotechnology (IMBB), Heraklion, Crete, Greece.
- 32 University of Crete, School of Medicine, Laboratory of Clinical Microbiology and Microbial Pathogenesis, Voutes, Heraklion, Crete, Greece.
- 33 The Institute of Cancer Research, Cancer Therapeutics Unit, Sutton, UK.
- 34 Pharmaceutical Sciences Department, College of Pharmacy, Gulf Medical University, Ajman, United Arab Emirates.
- 35 University of Sydney, Department of Pathology and Bosch Institute, Sydney, NSW, Australia.
- 36 Insight Institute of Neurosurgery & Neuroscience, Department of Research, Flint, MI, USA.
- 37 McGill University, Departments of Medicine and Oncology and Segal Cancer Centre, Montreal, Quebec, Canada.
- 38 Technical University Dresden, Center for Molecular and Cellular Bioengineering (CMCB), Biotechnology Center (BIOTEC), Department of Cellular Biochemistry, Dresden, Germany.
- 39 Universidad de Sevilla, Departamento de Psicología Experimental, Facultad de Psicología, Sevilla, Spain.
- 40 Sapienza University of Rome, Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, Rome, Italy.
- 41 The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- 42 University of Rajshahi, Department of Pharmacy, Rajshahi, Bangladesh.
- 43 Department of Biological Sciences and Biotechnology, Faculty of Sciences, Islamic University of Gaza, Gaza 108, Palestine.
- 44 Department of Human Anatomy and Cell Science, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada.
- 45 University of Barcelona, Faculty of Pharmacy, Department of Biochemistry and Physiology, Barcelona, Spain.
- 46 Metabolism and Cancer Laboratory, Molecular Mechanisms and Experimental Therapy in Oncology Program (Oncobell), Institut d'Investigació Biomèdica de Bellvitge - IDIBELL, L'Hospitalet de Llobregat, Barcelona, Spain.
- 47 The Francis Crick Institute, Molecular Cell Biology of Autophagy, London, UK.
- 48 Cleveland Clinic, Department of Cancer Biology, Cleveland, OH, USA.
- 49 University of the Basque Country, Instituto Biofisika (UPV/EHU, CSIC), and Department of Biochemistry and Molecular Biology, Leioa, Spain.
- 50 Instituto de Investigaciones en Ingeniería Genética y Biología Molecular "Dr. Héctor N. Torres", Laboratorio de señalización y mecanismos adaptativos en Tripanosomátidos, Buenos Aires, Argentina; Universidad de Buenos Aires, Departamento de Fisiología, Biología Molecular y Celular, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina.
- 51 Laboratory of Host-Pathogen Dynamics, National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
- 52 The Wistar Institute, Philadelphia, PA, USA.
- 53 Buenos Aires University, Biochemistry and Pharmacy School, Deparment of Microbiology Immunology Biotechnology and Genetic, IDEHU, CABA, Argentina.
- 54 Universidade do Porto, i3S-Instituto de Investigação e Inovação em Saúde, Porto, Portugal; IPATIMUP - Institute of Molecular Pathology and Immunology of the University of Porto, Cancer Drug Resistance Group, Porto, Portugal; FFUP - Faculty of Pharmacy of the University of Porto, Department of Biological Sciences, Porto, Portugal.
- 55 Université Côte d'Azur, INSERM, CNRS, IPMC, team labeled Laboratory of Excellence (LABEX) Distalz, Valbonne, France.
- 56 Islamic University of Gaza, Department of Medical Laboratory Sciences, Faculty of Health Sciences, Gaza, Palestine.
- 57 University of Pavia, Department of Drug Sciences, Pharmacology Unit, Pavia, Italy.
- 58 University of Camerino, School of Biosciences and Veterinary Medicine, Camerino, Italy.
- 59 University of Porto, Faculty of Pharmacy, UCIBIO, REQUIMTE, Porto, Portugal.
- 60 Telethon Institute of Genetics and Medicine (TIGEM), Pozzuoli, Naples, Italy.
- 61 Ohio State University, Department of Microbial Infection and Immunity, and the Infectious Disease Research Institute, Columbus, OH, USA.
- 62 Save as Ravi Manjithaya.
- 63 University of California, San Francisco, San Francisco, CA, USA.
- 64 Aarhus University, Department of Molecular Biology and Genetics, Aarhus C, Denmark.
- 65 Departments of Pharmacology and Toxicology, and Neurology, The University of Alabama at Birmingham, Birmingham, AL, USA.
- 66 Federal University of São Paulo, Department of Medicine, Nephrology Division, Laboratory of Clinical and Experimental Immunology, São Paulo, SP, Brazil.
- 67 Universidade Nove de Julho (UNINOVE), Faculty of Pharmacy, São Paulo, SP, Brazil.
- 68 Cedars-Sinai Medical Center, Smidt Heart Institute, Los Angeles CA, USA.
- 69 University of Bologna, Department of Pharmacy and Biotechnology, Bologna, Italy.
- 70 City of Hope Comprehensive Cancer Center, Beckman Research Institute, Department of Diabetes Complications and Metabolism, Duarte, CA, USA.
- 71 University of Tennessee, Department of Chemical and Biomolecular Engineering, Knoxville, TN, USA.
- 72 Northeast Ohio Medical university, Department of Anatomy & Neurobiology, Rootstown, OH, USA.
- 73 iNOVA4Health Chronic Diseases Research Center (CEDOC), Faculdade de Ciências Médicas, Universidade Nova de Lisboa, Lisboa, Portugal.
- 74 Max Planck Institute for Biology of Ageing and CECAD, University of Cologne, Germany.
- 75 School of Biochemistry, Faculty of Life Sciences, University of Bristol, Bristol, UK.
- 76 University of Helsinki, Molecular and Integrative Biosciences Research Programme, Helsinki, Finland.
- 77 INSERM, U1231, Dijon, France.
- 78 University of Valencia, Faculty of Medicine, Department of Pharmacology, Valencia, Spain; CIBERehd (Centre for Networked Biomedical Research in Hepatic and Digestive Diseases), Valencia, Spain.
- 79 National Institute of Neuroscience, National Center of Neurology and Psychiatry, Department of Peripheral Nervous System Research, Kodaira, Tokyo, Japan.
- 80 Osaka University, Graduate School of Dentistry, Osaka, Japan.
- 81 Tokyo University of Pharmacy and Life Sciences, School of Life Sciences, Hachioji, Horinouchi, Tokyo, Japan.
- 82 Universidade Federal de Viçosa, Departamento de Biologia Vegetal, Viçosa, MG, Brazil.
- 83 The Jikei University School of Medicine, Division of Respiratory Disease, Departent of Internal Medicine, Tokyo, Japan.
- 84 Newcastle University, Biosciences Institute, Newcastle Upon Tyne, UK.
- 85 Instituto Cajal, Consejo Superior de Investigaciones Científicas (CSIC) and CIBERFES, Madrid, Spain.
- 86 University of Seville, Faculty of Pharmacy, Department of Physiology, Seville, Spain.
- 87 Albert Einstein College of Medicine, Department of Medicine, Bronx, NY, USA.
- 88 Howard University College of Medicine, Department of Anatomy, Washington D.C., USA.
- 89 Tohoku University, Graduate School fo Life Sciences, Sendai, Miyagi, Japan.
- 90 University of Michigan, Life Sciences Institute, Ann Arbor, MI, USA.
- 91 Imperial College London, MRC Centre for Molecular Bacteriolgy and Infection, Department of Microbiology, London, UK.
- 92 Toulouse University, CNRS, UPS, Center for Integrative Biology (CBI), Research Center on Animal Cognition (CRCA), Toulouse, France.
- 93 Iowa State University, Department of Genetics, Development and Cell Biology, Roy J. Carver Co-Laboratory, Ames, IA, USA.
- 94 Universidad Nacional de Cordoba, Laboratorio de Oncohematología, Hospital Nacional de Clínicas, CONICET, Córdoba, Argentina.
- 95 York College/The City University of New York, Department of Biology, Jamaica, NY, USA.
- 96 Translational Genomics Group, Incliva Health Research Institute, Burjasot, Valencia, Spain; Interdisciplinary Research Structure for Biotechnology and Biomedicine (ERI BIOTECMED), University of Valencia, Burjasot, Valencia, Spain.
- 97 University of Palermo, Department of Biological, Chemical and and Pharmaceutical Sciences and Technologies, Palermo, Italy.
- 98 Albert Einstein College of Medicine, Department of Molecular Pharmacology, Bronx, NY, USA.
- 99 Faculty of Engineering and Natural Sciences, Sabanci University, Orta Mahalle, Üniversite Caddesi No. 27, Orhanlı, Tuzla, 34956 Istanbul, Turkey; Sabanci University Nanotechnology Research and Application Center (SUNUM), Tuzla, 34956, Istanbul, Turkey.
- 100 Meir Medical Center, Translational Hemato-Oncology Laboratory, Kfar-Saba, Israel; Tel Aviv University, Department of Human Molecular Genetics and Biochemistry, Sackler School of Medicine, Tel Aviv, Israel.
- 101 The Institute of Genetics and Animal Biotechnology, Polish Academy of Sciences, Magdalenka, Poland; Ludwig Boltzmann Institute for Digital Health and Patient Safety, Medical University of Vienna, Vienna, Austria; Institute of Neurobiology, Bulgarian Academy of Sciences, Sofia, Bulgaria; Department of Pharmacognosy, University of Vienna, Vienna, Austria.
- 102 University of Pittsburgh Medical Center, Department of Critical Care Medicine, Department of Pediatrics, Safar Center for Resuscitation Research and the Children's Hospital of Pittsburgh, Pittsburgh, PA, USA.
- 103 University of Nice, Mediterranean Center for Molecular Medicine, Inserm U1065, Nice, France.
- 104 Imperial College London, Faculty of Medicine, London, UK.
- 105 Departments of Pharmacology and Microbiology, University of Maryland School of Medicine, Baltimore, MD, USA; Stanford University School of Medicine Stanford, CA, USA.
- 106 University of Turin, Department of Clinical and Biological Sciences, Turin, Italy.
- 107 University of Milan, Department of Health Sciences, Milano, Italy.
- 108 Universidad de Santiago de Chile, Faculty of Chemistry and Biology, Department of Biology, Santiago, Chile.
- 109 Fondazione Istituto di Ricerca Pediatrica Città della Speranza, Neuroblastoma Laboratory, Padua, Italy.
- 110 CNC - Center for Neuroscience and Cell Biology, Coimbra, IIIUC - Instituto de Investigação Interdisciplinar, University of Coimbra, Coimbra, Portugal.
- 111 The Hebrew University of Jerusalem, Alexander Silberman Institute of Life Sciences, Department of Plant and Environmental Sciences, Edmond Safra Campus, Jerusalem, Israel.
- 112 Tulane Health Sciences Center, Department of Pathology and Laboratory Sciences, New Orleans, LA, USA.
- 113 Melbourne Dementia Research Centre, The Florey Institute of Neuroscience & Mental Health, The University of Melbourne, Parkville, VIC, Australia.
- 114 University of Arkansas for Medical Sciences, Department of Geriatrics, Little Rock, AR, USA.
- 115 University of Malta, Department of Physiology & Biochemistry, Msida, Malta.
- 116 Kogakuin University, Research Institute for Science and Technology, Hachioji, Tokyo, Japan.
- 117 Albert Einstein College of Medicine, Departments of Molecular Pharmacology and Biochemistry, Bronx, NY, USA.
- 118 Eastern Michigan University, Department of Chemistry, Ypsilanti, MI, USA.
- 119 Hanyang University, College of Pharmacy, Ansan, Gyeonggido, Korea.
- 120 Yonsei University College of Medicine, Severance Biomedical Science Institute, Seoul, Korea.
- 121 University of Massachusetts Medical School, Department of Molecular, Cell and Cancer Biology, Worcester, MA, USA.
- 122 Konkuk University, Department of Bioscience and Biotechnology, Seoul, Korea.
- 123 Ajou University, College of Pharmacy and Research Institute of Pharmaceutical Science and Technology (RIPST), Suwon, Korea.
- 124 Seoul National University, Department of Biological Sciences, Seoul, South Korea.
- 125 University of Calabria, Department of Pharmacy, Health Science and Nutrition, Section of Preclinical and Translational Pharmacology, Cosenza, Italy.
- 126 Adam Mickiewicz University/Faculty of Biology, Institute of Experimental Biology, Department of General Botany, Poznan, Poland.
- 127 Iowa State University, Department of Genetics, Development, and Cell Biology, Ames, IA, USA.
- 128 Kunming University of Science and Technology, Medical School, Kunming, Yunnan, China.
- 129 National Jewish Health, Department of Academic Affairs, Denver, CO, USA.
- 130 University of Texas Health Science Center at San Antonio, Department of Cell Systems and Anatomy, San Antonio, TX, USA.
- 131 Jadavpur University, Department of Mathematics, Centre for Mathematical Biology and Ecology, Kolkata, India.
- 132 Karolinska Institute, Department of Cell and Molecular Biology, Stockholm, Sweden.
- 133 University of Genoa, Department of Earth, Environment and Life Sciences, Genoa, Italy.
- 134 University of Siena, Department of Life Sciences, Siena, Italy.
- 135 University of Urbino Carlo Bo, Department of Biomolecular Sciences, Urbino (PU), Italy.
- 136 Telethon Institute of Genetics and Medicine (TIGEM), Naples, Italy; Baylor College of Medicine, Houston, TX, USA.
- 137 University of Naples Federico II, Department of Translational Medical Sciences, Naples, Italy.
- 138 Institute for Research and Technology in Food and Agriculture (IRTA), Animal Breeding and Genetics Programme, Caldes de Montbui, Spain.
- 139 Max Planck Institute of Molecular Plant Physiology, Potsdam-Golm, Germany.
- 140 UCL Queen Square Institute of Neurology, Reta Lila Weston Institute, London, UK.
- 141 Middle East Technical University, Department of Biological Sciences, Ankara, Turkey.
- 142 University of Miami, Department of Surgery and Sylvester Comprehensive Cancer Center, Miami, FL, USA.
- 143 University of Miami, Department of Surgery, Miami, FL, USA.
- 144 Université Côte D'Azur, CNRS, Inserm, Institut de Biologie Valrose, Nice, France.
- 145 School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China.
- 146 Universidade de São Paulo. Department of Biochemistry, Institute of Chemistry, Sao Paulo, Brazil.
- 147 University of Bologna, Department of Biomedical and Neuromotor Sciences, Bologna, Italy.
- 148 University of Rome Tor Vergata, Department of Biology, Rome, Italy; Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione Santa Lucia, Laboratory of Cell Signaling, Rome, Italy.
- 149 Edinburgh Napier University, School of Applied Sciences, Edinburgh, UK.
- 150 University of Michigan, Department of Neurology, Ann Arbor, MI, USA.
- 151 Pulmonology Department, Hospital del Mar-IMIM, Pompeu Fabra University, CIBERES, Barcelona, Spain.
- 152 University of Limerick, Department of Biological Sciences, Limerick, Ireland; University of Limerick, Health Research Institute, Limerick, Ireland.
- 153 Danish Cancer Society Research Center, Copenhagen, Denmark.
- 154 Rice University, Department of Biosciences, Houston, TX, USA.
- 155 Columbia University, Department of Medicine, New York, NY, USA.
- 156 Jawaharlal Nehru Centre for Advanced Scientific Research, Molecular Biology and Genetics Unit, and Neuroscience Unit, Autophagy Laboratory, Jakkur, Bangalore, India.
- 157 ICAR-Indian Veterinary Research Institute, FMD Vaccine Research Laboratory, Bengaluru, India.
- 158 The University of Texas M.D. Anderson Cancer Center, Department of Experimental Therapeutics, Houston, TX, USA.
- 159 University of North Texas Health Science Center, Department of Microbiology, Immunology & Genetics, Fort Worth, TX, USA.
- 160 University of Louvain, Louvain Institute of Biomolecular Science and Technology (LIBST), Louvain-la-Neuve, Belgium.
- 161 Trinity College Dublin, School of Medicine, Trinity Translational Medicine Institute, Dublin, Ireland.
- 162 Université Paris-Saclay, INSERM UMR1195, Hôpital Le Kremlin Bicêtre, 80 rue du Général Leclerc, 94276 Kremlin-Bicêtre, France.
- 163 University of South Carolina Upstate, Spartanburg, SC, USA.
- 164 Institut National de la Santé et de la Recherche Médicale (INSERM), Sorbonne Université, Université de Paris, Centre de Recherche des Cordeliers, Paris, France.
- 165 Francis Crick Institute, London, UK; UCL, Royal Free Hospital, London, UK.
- 166 Université Paris-Saclay, Inserm U1185, Le Kremlin-Bicêtre, France.
- 167 Université de Pau et des Pays de l'Adour, E2S UPPA, INRAE, NUMEA, 64310 Saint-Pée-sur-Nivelle, France.
- 168 University of Sao Paulo, Institute of Biomedical Sciences, Sao Paulo, SP, Brazil.
- 169 Emory University School of Medicine, Division of Endocrinology, Metabolism and Lipids, Atlanta, GA, USA.
- 170 University of Pennsylvania, Perelman School of Medicine, PENN Center for Pulmonary Biology, Lung Epithelial Biology Laboratories, Philadelphia, PA, USA.
- 171 Mater Research Institute, University of Queensland, Brisbane Australia.
- 172 Ludwig-Maximilians-Universität München, Munich Cluster for Systems Neurology, München, Germany.
- 173 Hannover Medical School, Department for Clinical Immunology and Rheumatology, Hannover, Germany.
- 174 University of Rome "Tor Vergata", Department of Clinical Sciences and Translational Medicine, Rome, Italy.
- 175 Tufts University, USDA Human Nutrition Research Center on Aging, Laboratory for Nutrition and Vision Research, Boston, MA, USA.
- 176 Universidad Cardenal Herrera-CEU, CEU Universities, School of Health Sciences, Department of Biomedical Sciences, Moncada, Spain.
- 177 Bar-Ilan University, Azrieli Faculty of Medicine, Safed, Israel.
- 178 University Medical Center of the Johannes Gutenberg University, Institute of Pathobiochemistry, Mainz, Germany.
- 179 Boston University, Metcalf Science Center, Boston, MA, USA.
- 180 Sorbonne Université, CNRS UMR8226, Institut de Biologie Physico-Chimique, Laboratoire de Biologie Moléculaire et Cellulaire des Eucaryotes, Paris, France.
- 181 Italian Liver Foundation, Trieste, Italy.
- 182 University of Rome "Sapienza", Department of Clinical and Molecular Medicine, Laboratory affiliated to Istituto Pasteur Italia Fondazione Cenci Bolognetti, Rome, Italy.
- 183 Centro Nacional de Biotecnología-CNB-CSIC, Madrid, Spain.
- 184 Universidad Autónoma de Madrid, Departamento de Biología Molecular, Madrid, Spain.
- 185 University of São Paulo, Faculty of Pharmaceutical Sciences, Department of Pathophysiology, Sao Paulo, Brazil.
- 186 Universidad Mayor, Center for Integrative Biology, Santiago, Chile.
- 187 University of Oklahoma Health Sciences Center/Stephenson Cancer Center, Department of Obstetrics and Gynecology, Section of Gynecologic Oncology, Oklahoma City, OK, USA.
- 188 UMR 7242 "Biotechnology and cellular signaling", CNRS, Illkirch, France.
- 189 Washington University in Saint Louis, School of Medicine, Department of Psychiatry, Saint Louis MO, USA.
- 190 Universidad Francisco de Vitoria, Madrid, Spain.
- 191 Hopwood Centre for Neurobiology, Lifelong Health Theme, South Australian Health and Medical Research Institute, Adelaide, Australia.
- 192 University of Copenhagen, Department of Biology, Copenhagen, Denmark.
- 193 University of Lausanne Hospital and Lausanne University, Institute of Pathology, Department of Laboratory Medicine and Pathology, Lausanne, Switzerland.
- 194 Queen Mary University of London, Blizard Institute, Centre for Cell Biology and Cutaneous Research, London, UK.
- 195 University of Cologne and University Hospital Cologne, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany; Center for Molecular Medicine, Cologne, Germany.
- 196 University of Massachusetts Medical School, Department of Molecular, Cell and Cancer Biology (MCCB), Worcester, MA, USA.
- 197 Magna Graecia University, Department of Health Sciences, Catanzaro, Italy.
- 198 Université Paris Descartes, Institut Cochin, INSERM U1016, CNRS UMR8104, Paris, France.
- 199 CNRS, Université de Bordeaux, Laboratoire de Biogenèse Membranaire UMR5200, 33140 Villenave d'Ornon, Bordeaux, France; Université de Bordeaux, CNRS, Laboratoire de Biogenèse Membranaire UMR5200, Villenave d'Ornon, Bordeaux, France.
- 200 Université du Québec à Trois-Rivières, Département de Biologie Médicale, Trois-Rivières, Québec, Canada.
- 201 University of Michigan, Department of Ophthalmology and Visual Sciences, Ann Arbor, MI, USA.
- 202 University of Montpellier, CNRS/UMR5235 LPHI, Montpellier, France.
- 203 Bristol Medical School, University of Bristol, Bristol, UK.
- 204 Ghent University, VIB Center for Inflammation Research, Ghent, Belgium.
- 205 University of Oxford, Kennedy Institute of Rheumatology, Oxford, UK.
- 206 University of New Mexico, Department of Molecular Genetics and Microbiology, Department of Neurology, Albuquerque, NM, USA.
- 207 Comenius University, Department of Biochemistry, Bratislava, Slovakia.
- 208 University of Oklahoma Health Sciences Center, Department of Obstetrics and Gynecology, Oklahoma City, OK, USA.
- 209 Vanderbilt University Medical Center, Vanderbilt Eye Institute, Nashville, TN, USA.
- 210 Department of Biophysics, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- 211 Louisiana State University Health Sciences Center-Shreveport, Department of Pathology and Translational Pathobiology, Shreveport, LA, USA.
- 212 National Institute of Technology Rourkela, Department of Life Science, Cancer and Cell Death Laboratory, Rourkela, Odisha, India.
- 213 Michigan Technological University, Department of Chemistry, Houghton, MI, USA.
- 214 Dalian Medical University, College of Basic Medical Sciences, Dalian, China.
- 215 Garvan Institute of Medical Research, St Vincent's Medical School, Faculty of Medicine, UNSW, Sydney, Australia.
- 216 Eötvös Loránd University (ELTE), Department of Genetics, Budapest, Hungary.
- 217 Federal University of São Paulo, Department of Pharmacology, Paulista School of Medicine, São Paulo, Brazil.
- 218 University Hospital of North Norway, The Heart and Lung Clinic; University of Tromso-The Artic University of Norway, Department of Clinical Medicine, Tromso, Norway.
- 219 Norwegian University of Science and Technology, Department of Biomedical Laboratory Science, Trondheim, Norway.
- 220 University of York, Department of Biology, Heslington, UK.
- 221 University of Valencia, Faculty of Medicine, Department of Physiology, Valencia, Spain; CIBERehd, Valencia, Spain.
- 222 University of Lodz, Faculty of Biology and Environmental Protection, Lodz, Poland.
- 223 Linköping University, Department of Biomedical and Clinical Sciences, Faculty of Medicine and Health Sciences, Linköping, Sweden.
- 224 Karolinska Institutet, Department of Women's and Children's Health, Stockholm, Sweden AND Karolinska University Hospital, Pediatric Oncology, Stockholm, Sweden.
- 225 Indiana University School of Medicine, Department of Microbiology and Immunology, Indianapolis, IN, USA.
- 226 St. Jude Children's Research Hospital, Department of Immunology, Memphis, TN, USA.
- 227 University of Zagreb, School of Medicine, Croatian Institute for Brain Research, Zagreb, Croatia.
- 228 University of Pennsylvania, Department of Basic and Translational Sciences, Philadelphia, PA, USA.
- 229 Burnet Institute, Melbourne, Australia.
- 230 University College Cork, Department of Pharmacology and Therapeutics, County Cork, Ireland.
- 231 INMG, INSERM, CNRS, University of Lyon, Lyon, France.
- 232 University of Padova, Department of Molecular Medicine, Padova, Italy.
- 233 Institut National de la Santé et de la Recherche Médicale, Centre de Recherche des Cordeliers, Sorbonne Université, Université de Paris, Paris, France.
- 234 National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Neurosciences and Cellular and Structural Biology Division (NCSBD), Bethesda, MD, USA.
- 235 West Virginia University, Department of Surgery, Morgantown, WV, USA.
- 236 The Open University, School of Life, Health and Chemical Sciences, Faculty of Science, Technology, Engineering and Mathematics, Milton Keynes, UK.
- 237 University of Rome Tor Vergata, Department of Biology, Rome, Italy.
- 238 IRCCS Bambino Gesù Children's Hospital, Department of Pediatric Hemato-Oncology and Cell and Gene Therapy, Rome, Italy.
- 239 Albert Ludwigs University of Freiburg, Faculty of Medicine, Institute of Molecular Medicine and Cell Research, Freiburg, Germany.
- 240 University Children's Hospital Zurich, Children's Research Center and Department of Oncology, Zurich, Switzerland.
- 241 University of Texas M.D. Anderson Cancer Center, Department of Leukemia, Houston, TX, USA.
- 242 Case Western Reserve University School of Medicine, Department of Pediatrics, Cleveland, OH, USA; InterRayBio, LLC, Baltimore, MD, USA.
- 243 Washington State University, Department of Veterinary Microbiology and Pathology, College of Veterinary Medicine, Pullman, WA, USA.
- 244 University Santiago de Compostela, Department of Pharmacology, Veterinary Faculty, Lugo, Spain.
- 245 Baylor College of Medicine, Jan and Dan Duncan Neurological Research Institute and Department of Molecular and Human Genetics, Houston, TX, USA.
- 246 Université de Paris, PARCC, INSERM, Paris, France.
- 247 University of Alabama at Birmingham, Department of Ophthalmology and Visual Sciences, Birmingham, AL, USA.
- 248 Albert Einstein College of Medicine, Department of Developmental and Molecular Biology, Bronx, NY, USA.
- 249 Medical University of Graz, Gottfried Schatz Research Center for Cell Signaling, Metabolism and Aging, Molecular Biology and Biochemistry, Graz, Austria.
- 250 University Paris 13, UMR_S942 Inserm; University of Paris, Bobigny, France.
- 251 Centro de Investigaciones Biológicas Margarita Salas, CSIC, Madrid, Spain.
- 252 Swedish University of Agricultural Sciences and Linnean Center for Plant Biology, Uppsala BioCenter, Department of Molecular Sciences, Uppsala, Sweden.
- 253 Stanford University, Department of Chemical & Systems Biology, Stanford, CA, USA.
- 254 Imperial College London, Department of Life Sciences, London, UK.
- 255 The Connecticut Agricultural Experiment Station, Center for Vector Biology and Zoonotic Diseases, Department of Environmental Sciences, New Haven, CT, USA.
- 256 Goethe University, University Hospital, University Cancer Center Frankfurt (UCT), Department of Medicine, Hematology/Oncology, Frankfurt, Germany.
- 257 Danube Private University, Department of Medicine/Dental Medicine, Krems/Donau, Austria.
- 258 Georg-August-Universität Göttingen, Institute for Microbiology and Genetics, Department of Molecular Microbiology and Genetics, Göttingen, Germany.
- 259 Universidad de Chile, Instituto de Nutrición y Tecnología de los Alimentos (INTA), Santiago, Chile.
- 260 Université de Paris, Sorbonne Université, Centre de Recherche des Cordeliers, INSERM U1138, Paris, France.
- 261 University Côte d'Azur (UCA), Institute for Research on Cancer and Aging of Nice (IRCAN), Centre National de la Recherche Scientifique (CNRS) UMR7284, Institut National de la Santé et de la Recherche Médicale (INSERM) U1081, Nice, France.
- 262 Centre des Sciences du Goût et de l'Alimentation, AgroSup Dijon, CNRS, INRAE, University of Bourgogne Franche-Comté, Eye and Nutrition Research Group, Dijon, France.
- 263 National Autonomous University of Mexico, Faculty of Chemistry, Department of Biology, Mexico city, Mexico.
- 264 University of California San Francisco, Department of Medicine, Zuckerberg San Francisco General Hospital and TraumaCenter, San Francisco, CA, USA.
- 265 University of Copenhagen, Department of Biology, Denmark.
- 266 University of Pittsburgh, Department of Biological Sciences, Pittsburgh, PA, USA.
- 267 Washington University School of Medicine, Department of Medicine, Saint Louis, MO, USA.
- 268 Department of Human Genetics, Genentech, Inc., South San Francisco, CA, USA.
- 269 David Geffen School of Medicine at UCLA, Department of Neurology, Los Angeles, CA, USA.
- 270 University of Colorado, AMC, Department of Pharmacology, Aurora, CO, USA.
- 271 University of Minnesota, Hormel Institute, Austin, MN, USA.
- 272 University of Sao Paulo, School of Physical Education and Sport, Cellular and Molecular Exercise Physiology Laboratory, Sao Paulo, SP, Brazil.
- 273 Hospital for Sick Children, Cell Biology Program, Toronto, ON, Canada.
- 274 University of Insubria, Department of Biotechnology and Life Sciences, Varese, Italy.
- 275 Monash University, School of Biological Sciences, Melbourne, Victoria, Australia.
- 276 University of Salento, Dept of Biological and Environmental Sciences and Technologies, Lecce, Italy.
- 277 Institut Pasteur, Biologie des Bactéries Intracellulaires and CNRS UMR 3525, Paris, France.
- 278 University of Pittsburgh, Aging Institute, Department of Medicine, Pittsburgh, PA, USA.
- 279 University Duisburg-Essen, Essen University Hospital, Department of Gastroenterology and Hepatology, Essen, Germany; Department of Gastroenterology, Hepatology & Endocrinology, Hannover Medical School, Hannover, Germany.
- 280 Thomas Jefferson University, Department of Pathology, Anatomy, and Cell Biology, Philadelphia, PA, USA.
- 281 Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA.
- 282 University of Arizona, Department of Molecular and Cellular Biology, Tucson, AZ, USA.
- 283 University of Iowa, Iowa City, IA, USA.
- 284 Montana BioAg. Inc. Missoula, MT, USA.
- 285 Universidad de Chile, Facultad de Odontología, Autophagy Research Center (ARC), Santiago, Chile.
- 286 KU Leuven, Department of Cellular & Molecular Medicine, Campus Gasthuisberg, Leuven, Belgium.
- 287 University of Medicine and Pharmacy of Craiova, Department of Medical Genetics, Craiova, Romania.
- 288 King's College London, School of Cardiovascular Medicine & Sciences, The Rayne Institute, St Thomas' Hospital, London, UK.
- 289 University of Cordoba, Department of Cell Biology, Physiology and Immunology, Córdoba, Spain.
- 290 The Rockefeller University, Laboratory of Cellular and Molecular Neuroscience, New York, NY, USA.
- 291 Stockholm University, The Wenner-Gren Institute, Department of Molecular Biosciences, Stockholm, Sweden.
- 292 University of Verona, Department of Neurosciences, Biomedicine and Movement Sciences, Biological Chemistry Section, Verona, Italy.
- 293 Departmento de Biología Celular e Histología, Facultad de Biología, Universidad de Murcia, Instituto Murciano de Investigación Biosanitaria (IMIB)-Arrixaca, Murcia, Spain.
- 294 Universidad Nacional Autónoma de México, Faculty of Sciences, Department of Cell Biology, Fibrosis Lab, Mexico City, Mexico.
- 295 New York University School of Medicine, Skirball Institute and Department of Microbiology, New York, NY, USA.
- 296 Wenzhou Medical University, State Key Laboratory of Ophthalmology, Optometry and Visual Science, School of Optometry and Ophthalmology and the Eye Hospital, Wenzhou, China.
- 297 University of Louisville, Pediatric Research Institute, Department of Pediatrics, Louisville, KY, USA.
- 298 Rutgers University, The State University of New Jersey, Department of Cell Biology and Neuroscience, Piscataway, NJ, USA.
- 299 University of Barcelona, Department of Biochemistry and Molecular Biomedicine, and Network Center for Biomedical Research in Pathophysiology of Obesity and Nutrition (CIBEROBN), Barcelona, Spain.
- 300 University of Las Palmas de Gran Canaria, Department of Physical Education and Research Institute of Biomedical and Health Sciences (IUIBS), Las Palmas de Gran Canaria, Spain; Norwegian School of Sport Sciences, Department of Physical Performance, Oslo, Norway.
- 301 University of Alabama, Department of Biological Sciences, Tuscaloosa, AL, USA.
- 302 University of Georgia, Department of Kinesiology, Athens, GA, USA.
- 303 Fondazione Policlinico Universitario "Agostino Gemelli" IRCCS, Rome, Italy.
- 304 University of Zaragoza, Faculty of Veterinary-IIS Aragón, IA2-CITA, CIBERNED, Laboratory of Genetics and Biochemistry (LAGENBIO), Zaragoza, Spain.
- 305 University of São Paulo, Department of Immunology, Laboratory of Transplantation Immunobiology, São Paulo, SP, Brazil.
- 306 CNRS, Institut Curie, Paris, France.
- 307 University of Bordeaux, CNRS, IBGC, UMR 5095, Bordeaux, France.
- 308 University of London, The Royal Veterinary College, Department of Comparative Biomedical Sciences, UCL Consortium for Mitochondrial Research, London, UK.
- 309 Loyola University, Stritch School of Medicine, Department of Microbiology and Immunology, Chicago, IL, USA.
- 310 University of Ottawa, Department of Chemistry and Biomolecular Sciences and Department of Biochemistry, Microbiology and Immunology, Host-Microbe Interactions Laboratory, Ottawa, ON, Canada.
- 311 University of Rome "Tor Vergata", Department of Biology, Rome, Italy.
- 312 Università di Sassari, Dipartimento di Scienze Biomediche, Sassari, Italy.
- 313 Université Côte d'Azur, Université Nice Sophia Antipolis, CEA/DRF/BIAM, Faculté de Médecine, UMR E-4320 TIRO-MATOs, Nice, France.
- 314 Universidad San Sebastián, Centro de Biología Celular y Biomedicina (CEBICEM), Facultad de Medicina y Ciencia, Santiago, Chile.
- 315 University of Toronto, Sinai Health System, Lunenfeld-Tanenbaum Research Institute, Toronto, Ontario, Canada.
- 316 IRBLleida-University of Lleida,Department of Experimental Medicine, Lleida, Spain.
- 317 Xiamen University School of Medicine, Fujian Provincial Key Laboratory of Reproductive Health Research, Xiang'an, Xiamen, China.
- 318 University of Campania "L. Vanvitelli", Department of Precision Medicine, Naples, Italy; Institute of Genetic Research, Biogem scarl, Laboratory of Molecular and Precision Oncology, Ariano Irpino (AV), Italy.
- 319 Instituto de Investigación Biomédica de A Coruña (INIBIC), Complexo Hospitalario Universitario de A Coruña (CHUAC), Sergas, Universidade da Coruña (UDC), A Coruña, Spain.
- 320 University of Wisconsin, Department of Surgery, Madison, WI, USA.
- 321 University of Geneva, Department of Biochemistry, Faculty of Science, Switzerland.
- 322 Universidad Nacional Autónoma de México, Institute of Biotechnology, Plant Molecular Biology Department, Cuernavaca, Morelos, México.
- 323 University of Coimbra, Faculty of Medicine and CNC-Center for Neuroscience and Cell Biology, Coimbra, Portugal.
- 324 University of Arizona Cancer Center, Tucson, AZ, USA.
- 325 Université Côte d'Azur, Université Nice Sophia Antipolis, CEA/DRF/Institut Frédéric Joliot, Faculté de Médecine, UMR E-4320 TIRO-MATOs, Nice, France.
- 326 Trev and Joyce Deeley Research Centre, BC Cancer, Victoria, British Columbia, Canada.
- 327 University of Graz, Institute of Molecular Biosciences, NAWI Graz, Graz, Austria.
- 328 Federal University of Rio de Janeiro, Institute of Microbiology, Department of Immunology, Rio de Janeiro, Brazil.
- 329 Polytechnic University of Marche, Department of Life and Environmental Sciences, Ancona, Italy.
- 330 University of Modena and Reggio Emilia, Department of Biomedical, Metabolic and Neural Sciences, Modena, Italy.
- 331 Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR7258, Aix-Marseille Université U105, Institut Paoli-Calmettes, Parc Scientifique et Technologique de Luminy, Marseille, France.
- 332 Functional Genomics of Cardiomyopathies, Institute of Experimental Pharmacology and Toxicology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
- 333 University of Alabama at Birmingham, Department of Medicine, Pulmonary, Allergy, and Critical Care Medicine, Birmingham VA Medical Center, Birmingham, AL, USA.
- 334 Universidade de Lisboa, Faculty of Pharmacy, Research Institute for Medicines (iMed.ULisboa), Lisboa, Portugal.
- 335 Aix Marseille Univ, CNRS, LISM, Institut de Microbiologie de la Méditerranée, Marseille, France.
- 336 Universitat Autònoma de Barcelona, Institute of Neuroscience, Barcelona, Spain.
- 337 Institute for Advanced Chemistry of Catalonia (IQAC-CSIC), Department of Biological Chemistry; Networking Biomedical Research Centre on Hepatic and Digestive Diseases (CIBER-EHD), Barcelona, Spain.
- 338 University of New Mexico Health Sciences Center, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Albuquerque, NM, USA.
- 339 Universidad de Valparaíso, Centro Interdisciplinario de Neurociencia de Valparaíso, Laboratory of Molecular Sensors, Valparaíso, Chile.
- 340 Complutense University, Instituto de Investigaciones Sanitarias San Carlos (IdISSC), Department of Biochemistry and Molecular Biology, School of Biology, Madrid, Spain.
- 341 Université de Paris, Sorbonne Université, Centre de Recherche des Cordeliers, INSERM U1138, Team "Metabolism, Cancer & Immunity", Paris, France; Gustave Roussy Cancer Campus, Metabolomics and Cell Biology Platforms, Villejuif, France.
- 342 Division of Medical Genetics, Fondazione IRCCS-Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy.
- 343 Universidad de Concepción, Department of Biochemistry and Molecular Biology, Concepción, Chile.
- 344 Universidade NOVA de Lisboa, Faculdade de Ciências e Tecnologia, Departamento Ciências da Vida, UCIBIO, Caparica, Portugal.
- 345 Universidad de La Laguna, Departamento de Ciencias Médicas Básicas, Tenerife, Spain.
- 346 Universidad Nacional Autónoma de México (UNAM), Instituto de Fisiologia Celular, Ciudad Universitaria, Ciudad de México, México.
- 347 The Scripps Research Institute, Department of Molecular Medicine, La Jolla, CA, USA.
- 348 University of Coimbra, Portugal, CNC - Center for Neuroscience and Cell Biology & Faculty of Pharmacy, Coimbra, Portugal.
- 349 University of Pisa, Interdepartmental Research Centre on Biology and Pathology of Aging, Pisa, Italy.
- 350 University of Innsbruck, Institute for Biomedical Aging Research, Department of Molecular Biology, Innsbruck, Austria.
- 351 Biomedical Research Institute of Murcia, IMIB-Arrixaca, Telomerase, Cancer and Aging Group, Surgical Department, Murcia, Spain.
- 352 Research Center Borstel, Leibniz Lung Center, Priority Area Infections, Division Cellular Microbiology, Borstel, Germany.
- 353 Danish Cancer Society Research Center, Center for Autophagy, Recycling and Disease (CARD), Cell Stress and Survival Unit, Copenhagen, Denmark; IRCCS Bambino Gesù Children's Hospital, Department of Pediatric Hemato-Oncology and Cell and Gene therapy, Rome, Italy; University of Rome Tor Vergata, Department of Biology, Rome, Italy.
- 354 Medical University of Bialystok, Department of Pharmaceutical Biochemistry, Bialystok, Poland.
- 355 Ospedale San Raffaele and Vita-Salute San Raffaele University, Division of Genetics and Cell Biology, Milano, Italy.
- 356 Hospital Universitari de Tarragona Joan XXIII, Institut d'Investigació Sanitària Pere Virgili, Madrid, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
- 357 University of Minho, School of Medicine, Life and Health Sciences Research Institute (ICVS), Braga, Portugal.
- 358 Universidade Federal de São Paulo, Department of Morphology and Genetics, São Paulo, SP, Brazil.
- 359 University of Tuscia, Department for Innovation in Biological, Agro-food, and Forest systems (DIBAF), Viterbo, Italy.
- 360 Ege University, Faculty of Medicine, Department of Medical Biology, Izmir, Turkey.
- 361 School of Pharmacy, Jeonbuk National University, Jeollabuk-do, Republic of Korea.
- 362 Karolinska Institutet, Department of Physiology and Pharmacology, Stockholm, Sweden.
- 363 Kaohsiung Medical University, Department of Pathology, Kaohsiung City, Taiwan.
- 364 University of Calcutta, Department of Biotechnology and Dr. B.C. Guha Centre for Genetic Engineering and Biotechnology, Kolkata, India.
- 365 Saha Institute of Nuclear Physics, Biophysics & Structural Genomics Division, HBNI, Kolkata, India.
- 366 Ehime University, Department of Aquatic Life Sciences, Ainan, Ehime, Japan.
- 367 Justus-Liebig-University Giessen, Institute of Medical Microbiology, Giessen, Germany.
- 368 The University of Hong Kong, Li Ka Shing Faculty of Medicine, Department of Obstetrics and Gynecology, Hong Kong, China.
- 369 Queen's University, Department of Biomedical and Molecular Sciences, Kingston, Ontario, Canada.
- 370 The Chinese University of Hong Kong, School of Life Sciences, Shatin N.T., Hong Kong, China.
- 371 The Chinese University of Hong Kong, School of Biomedical Sciences, Hong Kong, China.
- 372 The Chinese University of Hong Kong, Department of Anaesthesia and Intensive Care, Hong Kong, China.
- 373 Tulane University School of Medicine, Department of Pharmacology, New Orleans, LA, USA.
- 374 National Cheng Kung University, College of Medicine, Department of Microbiology and Immunology, Tainan, Taiwan.
- 375 University of California, Berkeley, Department of Molecular Biology and Cell Biology, Berkeley, CA, USA; University of California, Berkeley, California Institute for Quantitative Biosciences, Berkeley, CA, USA.
- 376 National University of Singapore, Faculty of Sciences, Department of Pharmacy, Singapore.
- 377 Southeast University, School of Medicine, Department of Physiology, Nanjing, Jiangsu, China.
- 378 University of East Anglia, School of Biological Sciences, Norwich Research Park, UK.
- 379 Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), INSERM U964, CNRS UMR7104, University of Strasbourg, Illkirch, France.
- 380 NCR Biotech Science Cluster, Translational Health Science and Technology Institute, Complex Analysis Group, Faridabad, India.
- 381 Banaras Hindu University, Institute of Science, Department of Zoology, Lanka, Varanasi, Uttar Pradesh, India.
- 382 University of Washington, Department of Laboratory Medicine, Seattle, WA, USA.
- 383 Institute of Life Sciences, Bhubaneswar, Odisha, India.
- 384 UCL Institute of Ophthalmology, London, UK.
- 385 National Cheng Kung University, Department of Biochemistry and Molecular Biology, Tainan, Taiwan.
- 386 Academia Sinica, Institute of Biological Chemistry, Taipei, Taiwan.
- 387 University of Southern California, Center for Craniofacial Molecular Biology, Los Angeles, CA, USA.
- 388 University of Minnesota Medical School, Department of Laboratory Medicine and Pathology, Minneapolis, MN, USA.
- 389 Qingdao University, Institute of Brain Science and Disease, Qingdao, Shandong, China.
- 390 Army Medical University (Third Military Medical University), Daping Hospital, Center of Bone Metabolism and Repair, State Key Laboratory of Trauma, Burns and Combined Injury, Trauma Center, Research Institute of Surgery, Laboratory for the Prevention and Rehabilitation of Training Related Injuries, Chongqing, China.
- 391 Third Military Medical University, Southwest Hospital, Institute of Pathology and Southwest Cancer Centre, Chongqing, China.
- 392 Changhua Christian Hospital, Department of Otorhinolaryngology-Head and Neck Surgery, Changhua, Taiwan.
- 393 Wuhan Sports University, College of Health Science, Tianjiu Research and Development Center for Exercise Nutrition and Foods, Hubei Key Laboratory of Exercise Training and Monitoring, Wuhan, Hubei, China.
- 394 College of Life Sciences, Nankai University, Tianjin, China.
- 395 Wuhan University, School of Pharmaceutical Sciences, Zhongnan Hospital, Wuhan, China.
- 396 Duke University, Department of Medicine, Durham, NC, USA.
- 397 Chinese Academy of Sciences, Institute of Modern Physics, Lanzhou, China.
- 398 University of Sydney, Sydney Medical School, Renal Medicine, St Leonards, NSW, Australia.
- 399 Temple University, Lewis Katz School of Medicine, Cardiovascular Research Center and Department of Physiology, Philadelphia, PA, USA.
- 400 Chinese Academy of Medical Sciences and Peking Union Medical College, Institute of Dermatology, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Nanjing, Jiangsu, China.
- 401 Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, Shanghai, China.
- 402 Tsinghua University, School of Life Sciences, Beijing, China.
- 403 Peking University School of Basic Medical Science, Department of Immunology, Beijing, China.
- 404 Research Institute in Oncology and Hematology, CancerCare Manitoba, Winnipeg, Manitoba, Canada.
- 405 Mackay Memorial Hospital, Department of Radiation Oncology, Taipei, Taiwan.
- 406 Sun Yat-sen University, School of Life Science, Guangzhou, China.
- 407 Wuhan University, School and Hospital of Stomatology, Key Lab of Oral Biomedicine of Ministry of Education (KLOBME), Wuhan, Hubei, China.
- 408 2nd Affiliated Hospital of Zhejiang University School of Medicine, Department of Respiratory and Critical Care Medicine, Hangzhou, China.
- 409 University of Texas Southwestern Medical Center, Department of Molecular Biology, Dallas, Texas, USA.
- 410 Purdue University, Department of Botany and Plant Pathology, West Lafayette, IN, USA.
- 411 Wuhan University, Hubei Key laboratory of Cell Homeostasis, College of Life Sciences, Wuhan, China.
- 412 Capital Medical University, Beijing Ditan Hospital, Liver Disease Center, Beijing, China.
- 413 Northwestern University Feinberg School of Medicine, Department of Neurology & Lou & Jean Malnati Brain Tumor Institute, Chicago, IL, USA.
- 414 Albert Einstein College of Medicine, Department of Genetics, Bronx, NY, USA.
- 415 The University of Texas Health Science Center at Houston, Department of Integrative Biology and Pharmacology, Houston, TX, USA.
- 416 National Institutes of Health, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
- 417 East China Normal University, School of Life Sciences, Shanghai, China.
- 418 University of Florida, Food Science and Human Nutrition Department, Gainesville, FL, USA.
- 419 Zhejiang University, College of Pharmaceutical Sciences, Institute of Pharmacology and Toxicology, Hangzhou, Zhejiang, China.
- 420 National Cancer Center Korea, Goyang, Gyeonggi, Korea.
- 421 National University of Singapore, Yong Loo Lin School of Medicine, Department of Biochemistry, Singapore.
- 422 National University of Singapore, Yong Loo Lin School of Medicine, Strategic Research Programme in Precision Medicine, Singapore.
- 423 Lomonosov Moscow State University, A.N. Belozersky Institute of Physico-Chemical Biology, Moscow, Russia.
- 424 University of Pennsylvania, Department of Pathology and Laboratory Medicine, Philadelphia, PA, USA.
- 425 National Cheng Kung University, College of Medicine, Department of Pharmacology, Tainan, Taiwan.
- 426 Sun Yat-Sen University, School of Pharmaceutical Sciences, Guangzhou, China.
- 427 INSERM U1242, University of Rennes, Rennes, France; Centre de Lutte Contre le Cancer Eugène Marquis, Rennes, France.
- 428 University of North Carolina at Charlotte, Department of Biological Sciences, Charlotte, NC, USA.
- 429 The University of Hong Kong, Queen Mary Hospital, Department of Paediatrics and Adolescent Medicine, Pokfulam, Hong Kong, China.
- 430 University of Milano-Bicocca, Department of Biotechnology and Biosciences, Milan, Italy.
- 431 Istituto di Fisiologia Clinica, Consiglio Nazionale delle Ricerche, Siena, Italy; Core Research Laboratory, Istituto per lo Studio, la Prevenzione e la Rete Oncologica, Siena, Italy.
- 432 The Norwegian Radium Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Montebello, Oslo, Norway; University of Oslo, Faculty of Mathematics and Natural Sciences, The Department of Biosciences, Oslo, Norway.
- 433 The Norwegian Radium Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Montebello, Oslo, Norway; University of Oslo, Faculty of Medicine, Institute of Clinical Medicine, Centre for Cancer Cell Reprogramming, Oslo, Norway.
- 434 IEO, European Institute of Oncology IRCCS, IFOM-IEO Campus, Department of Experimental Oncology, Milan, Italy.
- 435 University of Chile, Faculty of Chemical and Pharmaceutical Sciences, Advanced Center for Chronic Diseases (ACCDiS), Santiago, Chile.
- 436 Taipei Veterans Generals Hospital, Department of Medical Research, Taipei, Taiwan.
- 437 National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Rocky Mountain Laboratories (RML), Laboratory of Virology, Innate Immunity and Pathogenesis Section, Hamilton, MT, USA.
- 438 Institute of Translational Pharmacology, National Research Council, Rome, Italy; European Center for Brain Research, Laboratory of Resolution of Neuroinflammation, IRCCS Santa Lucia Foundation, Rome, Italy.
- 439 Kyungpook National University, Department of Life Science, Deagu, Korea.
- 440 Wonkwang University School of Medicine, Department of Microbiology, Iksan, Jeonbuk, Korea.
- 441 Weill Cornell Medicine, Department of Medicine, New York, NY, USA.
- 442 Weill Cornell Medicine, Division of Nephrology and Hypertension, Joan and Sanford I. Weill Department of Medicine, New York, NY, USA.
- 443 CSIR-Indian Institute of Chemical Biology, Kolkata, India.
- 444 BC Cancer Research Centre, Department of Experimental Therapeutics, Vancouver, BC, Canada.
- 445 University of Pittsburgh School of Medicine, Department of Pathology, Pittsburgh, PA, USA.
- 446 National University of Singapore, Yong Loo Lin School of Medicine, Department of Physiology; National University of Singapore, LSI Neurobiology Programme; National University of Singapore, Institute for Health Innovation and Technology; National University Hospital Singapore, Centre for Healthy Longevity; Institute of Molecular and Cell Biology, Agency for Science, Technology and Research (A*STAR), Singapore.
- 447 Konkuk University School of Medicine, Department of Ophthalmology, Seoul, Korea.
- 448 Max-Planck-Institut für Molekulare Pflanzenphysiologie, Potsdam, Germany.
- 449 University of Ulsan College of Medicine, Asan Medical Center, Department of Psychiatry, Seoul, Korea.
- 450 The Catholic University of Korea, Seoul St, Mary's Hospital, Department of Ophthalmology and Visual Science, Seoul, Kor.
- 451 Institute of Cancer Research, Cancer Research UK Cancer Imaging Centre, Division of Radiotherapy and Imaging, London, UK.
- 452 Nencki Institute of Experimental Biology of Polish Academy of Sciences, Neurobiology Center, Laboratory of Molecular Neurobiology, Warsaw, Poland.
- 453 University of Chile, Institute of Nutrition and Food Technology (INTA), Advanced Center for Chronic Diseases (ACCDiS), Santiago, Chile.
- 454 University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Pediatrics, Cologne, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Center for Molecular Medicine (CMMC), Cologne, Germany.
- 455 University of Rome "La Sapienza", Rome, Italy.
- 456 University of Liverpool, Institute of Translational Research, Cellular and Molecular Physiology, Liverpool, UK.
- 457 Molecular Physiology and Cell Signaling, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, UK.
- 458 Università di Milano, Department of Biomedical and Clinical Sciences L. Sacco, Milan, Italy.
- 459 Istituto Superiore di Sanità, Department of Infectious Diseases, Rome, Italy.
- 460 Institut Necker-Enfants Malades (INEM), INSERM U1151/CNRS UMR 8253, Université de Paris, Paris, France.
- 461 The Hebrew University of Jerusalem, The Institute for Medical Research Israel-Canada, Department of Biochemistry and Molecular Biology, Ein Karem, Jerusalem, Israel.
- 462 University Roma Tre, Department of Science, LIME, Rome, Italy.
- 463 National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Pediatric Translational Research Branch, Bethesda, MD, USA.
- 464 Institut d'Investigacions Biomèdiques de Barcelona (CSIC, IDIBAPS), and Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Barcelona, Spain.
- 465 University of Belgrade, Institute for the Application of Nuclear Energy (INEP), Belgrade, Serbia.
- 466 Centre for Research in Agricultural Genomics (CRAG), CSIC-IRTA-UAB-UB, Campus UAB, Bellaterra, Barcelona, Spain.
- 467 University of Sheffield, Department of Biomedical Science, Firth Court, Western Bank, Sheffield, UK.
- 468 Instituto de Histología y Embriología (IHEM)-Universidad Nacional de Cuyo-CONICET, Facultad de Ciencias Médicas, Mendoza, Argentina.
- 469 Yale University School of Medicine, Department of Neuroscience and Department of Cell Biology, New Haven, CT, USA.
- 470 Clermont-Auvergne University, INRAE, Human Nutrition Unit, F-63000, Clermont-Ferrand, France.
- 471 University of Pavia, Department of Biology and Biotechnology "L. Spallanzani", Pavia, Italy.
- 472 Federal University of São Carlos, Department of Gerontology, São Carlos, SP, Brazil.
- 473 Department of Pathology and Experimental Therapeutics and Institute of Biomedicine of the University of Barcelona (IBUB), Bellvitge University Hospital-IDIBELL, Hospitalet de Llobregat; University of Brescia, Department of Molecular and Translational Medicine, Brescia BS, Italy.
- 474 National Institutes of Health, NIAMS, Lymphocyte Nuclear Biology, Bethesda, MD, USA.
- 475 Rutgers University, Department of Biological Sciences, Newark, NJ, USA.
- 476 National Institutes of Health, National Institute on Aging, Laboratory of Neurogenetics, Bethesda, MD, USA.
- 477 University of Manitoba, Department of Medical Microbiology & Infectious Diseases, Winnipeg, Manitoba, Canada.
- 478 Johns Hopkins University, Department of Molecular Microbiology and Immunology, Baltimore, MD, USA.
- 479 University Magna Graecia of Catanzaro, Department of Health Sciences, Catanzaro, Italy.
- 480 Umeå University, Umeå Centre for Microbial Research (UCMR), Department of Chemistry, Umeå, Sweden.
- 481 OncoRay National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany; Helmholtz-Zentrum Dresden - Rossendorf, Institute of Radiooncology - OncoRay, Dresden, Germany; German Cancer Consortium (DKTK), Partner site Dresden, Germany; German Cancer Research Center (DKFZ), Heidelberg, Germany.
- 482 OncoRay National Center for Radiation Research in Oncology, Faculty of Medicine, Technische Universität Dresden, Dresden, Germany.
- 483 University of Genoa, DIMES Department of Experimental Medicine, Human Anatomy, Genoa, Italy.
- 484 University of Michigan Medical School, Department of Neurology, Ann Arbor, MI, USA.
- 485 University of Zürich, Schlieren Campus, Center for Molecular Cardiology, Schlieren, Switzerland.
- 486 University of Oviedo, Department of Morphology and Cell Biology, Oviedo, Spain.
- 487 University of Melbourne, Department of Pharmacology and Therapeutics, Melbourne, Victoria, Australia.
- 488 CSIC-Universidad de Sevilla, Instituto de Bioquímica Vegetal y Fotosíntesis, Sevilla, Spain.
- 489 Universidad de Chile, Facultad de Odontología, Advanced Center for Chronic Diseases, Santiago, Chile.
- 490 Università degli Studi di Milano, Dipartimento di Scienze Farmacologiche e Biomolecolari, Centro di Eccellenza per lo studio delle Malattie Neurodegenerative, Milano, Italy.
- 491 Department of Anatomy, Cell and Developmental Biology, Eötvös Loránd University, Budapest, Hungary.
- 492 Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Instituto de Investigación Sanitaria La Paz (IdiPaz), Instituto de Investigaciones Biomédicas Alberto Sols UAM-CSIC, Madrid, Spain; Universidad Autónoma de Madrid, School of Medicine, Department of Biochemistry, Madrid, Spain.
- 493 Chinese Academy of Medical Sciences & Peking Union Medical College, Institute of Materia Medica, State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Molecular Immunology and Cancer Pharmacology Group, Beijing, China.
- 494 Sun Yat-sen University, School of Life Sciences, MOE Key Laboratory of Gene Function and Regulation, Guangzhou, Guangdong, China.
- 495 Medical Research Institute, Wuhan University, Wuhan, China.
- 496 The Chinese University of Hong Kong, School of Life Sciences, Centre for Cell & Developmental Biology and State Key Laboratory of Agrobiotechnology, Shatin, New Territories, Hong Kong, China.
- 497 Université Paris-Saclay, CEA, 1035 CNRS, Institute for Integrative Biology of the Cell (I2BC), INSERM U1280, Gif-sur-Yvette, France.
- 498 University of Mar del Plata, Department of Biology and Chemestry, Mar del Plata, BA, Argentina.
- 499 McGill University Health Centre, Montréal, QC, Canada.
- 500 Emory University School of Medicine, Department of Medicine, Atlanta, GA, USA.
- 501 Medical University of Lublin, Department of Pathophysiology, Lublin, Poland.
- 502 University of Bologna, Department of Medical and Surgical Sciences; Rizzoli Orthopaedics Institute, Laboratory of Immunorheumatology and Tissue Regeneration, Bologna, Italy.
- 503 University Campus Bio-Medico, Department of Medicine, Rome, Italy.
- 504 Istituto Dermopatico dell'Immacolata, IDI-IRCCS, Rome, Italy.
- 505 University of Florida, Department of Physical Therapy, Gainesville, FL, USA.
- 506 University "G. D'Annunzio", Department of Medical Sciences DSMOB, Chieti, Italy; Regina Elena National Cancer Institute, Department of Research, Rome, Italy.
- 507 Federal University of Sergipe, Department of Pharmacy, São José, SE, Brazil.
- 508 University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Pediatrics, Cologne, Germany.
- 509 University of Nevada, Reno School of Medicine, Department of Pharmacology, Reno, NV, USA.
- 510 Gregor Mendel Institute, Vienna Biocenter, Vienna, Austria.
- 511 University of Naples Federico II, Department of Pharmacy, Naples, Italy.
- 512 Xi'an Jiaotong University, Department of Public Health, Xi'an, Shaanxi, China.
- 513 The First Hospital of Jilin University, Laboratory of Cancer Precision Medicine, Changchun, Jilin, China.
- 514 Model Animal Research Center of Nanjing University, Nanjing, Jiangsu, China.
- 515 University of Salerno, Department of Medicine, Surgery and Dentistry "Scuola Medica Salernitana", Baronissi, SA, Italy.
- 516 University of Padova, Department of Biology, Padova, Italy.
- 517 Research Center Borstel, Cellular Microbiology, Borstel, Germany.
- 518 University of Clermont Auvergne, M2iSH (Microbes, Intestine, Inflammation and Susceptibility of the Host), UMR 1071 Inserm, INRA USC 2018, CRNH, Clermont-Ferrand, France.
- 519 Christian-Albrechts-University Kiel, Biochemical Institute, Kiel, Germany.
- 520 Karolinska Institutet, Biomedicum, Department of Cell and Molecular Biology, Stockholm, Sweden.
- 521 University of South Florida, Morsani College of Medicine, Department of Molecular Medicine and USF Health Byrd Alzheimer's Research Institute, Tampa, FL, USA; Institute for Biological Instrumentation of Russian Academy of Sciences, Laboratory of New Methods in Biology, Pushchino, Moscow region, Russia.
- 522 University of South Florida, Morsani College of Medicine, Department of Molecular Medicine, Tampa, FL, USA.
- 523 Texas Tech University Health Sciences Center, Department of Pharmaceutical Sciences, Amarillo, TX, USA.
- 524 Cleveland Clinic, Department of Gastroenterology, Hepatology and Nutrition, Cleveland, OH, USA.
- 525 MD Anderson Cancer Center, Department of Gynecologic Oncology and Reproductive Medicine, Houston, TX, USA.
- 526 Tulane University Health Sciences Center, Department of Pathology and Laboratory Medicine, New Orleans, LA, USA.
- 527 Max-Delbrück-Center for Molecular Medicine, Department of Crystallography, Berlin, Germany.
- 528 Institute of Life Sciences, School of Medicine, Swansea University, Swansea, Wales, UK.
- 529 University of Massachusetts Medical School, Program in Molecular Medicine, Worcester, MA, USA.
- 530 Stellenbosch University, Department of Physiological Sciences, Stellenbosch, Western Cape, South Africa.
- 531 University of Dundee School of Medicine, Division of Cellular Medicine, Dundee, Scotland, UK.
- 532 University of Calabria, Department of Farmacy Health and Nutritional Sciences, Rende, Italy.
- 533 Ghent University, Translational Nuclear Receptor Research, VIB Center for Medical Biotechnology, Department of Biomolecular Medicine, Ghent, Belgium.
- 534 "Sapienza" University of Rome, Department of Radiotherapy, Policlinico Umberto I, Rome, Italy.
- 535 University of Verona, Azienda Ospedaliera Universitaria Integrata-Verona, Department of Medicine, Verona; Italy.
- 536 Oswaldo Cruz Foundation, Oswaldo Cruz Institute, Leprosy Laboratory, Rio de Janeiro, Brazil.
- 537 University of Antwerp, Laboratory of Physiopharmacology, Antwerp, Belgium.
- 538 Department of Oncology-Pathology, Cancer Center Karolinska, Karolinska Institute, Stockholm, Sweden.
- 539 Sapienza University of Rome, Ospedale Sant'Andrea, Rome, Italy.
- 540 Università degli Studi di Milano, Department of Medical Biotechnology and Translational Medicine, Milano, Italy.
- 541 University of Urbino Carlo Bo, Department of Biomolecular Sciences, Unit of Pharmacology and Public Health, Urbino, Italy.
- 542 University of Fribourg, Department of Biology, Fribourg, Switzerland.
- 543 Danish Cancer Society Research Center, Cell Stress and Survival Unit, Center for Autophagy, Recycling and Disease (CARD), Copenhagen, Denmark.
- 544 University of California, San Francisco, Department of Pathology, San Francisco, CA, USA.
- 545 Washington University School of Medicine, Departments of Pediatrics and Cell Biology & Physiology, St. Louis, MO, USA.
- 546 KU Leuven, Department of Development and Regenaration, Laboratory of Pediatrics, PKD Research Group, Leuven, Belgium.
- 547 Boston University, Department of Biology, Boston, MA, USA.
- 548 IFOM, The FIRC Institute of Molecular Oncology, Milan, Italy.
- 549 University of Colorado Anschutz Medical Campus, Department of Biochemistry and Molecular Genetics, Aurora, CO, USA.
- 550 University of Bordeaux, CNRS, IMN, UMR 5293, Bordeaux, France.
- 551 Instituto de Fisiologia Celular, UNAM, Department of Biochemistry and Structural Biology, Mexico City, Mexico.
- 552 Medical University of South Carolina, Charleston, SC 29425, USA.
- 553 University of Melbourne, School of Biomedical Sciences, Melbourne, Victoria, Australia.
- 554 University of Buenos Aires, Faculty of Pharmacy and Biochemistry, Institute of Immunology, Genetics and Metabolism (INIGEM), CONICET. Buenos Aires, Argentina.
- 555 L.N.C.I.B. Laboratorio Nazionale Consorzio Interuniversitario Biotecnologie, AREA Science Park, Trieste, Italy.
- 556 University of Zagreb School of Medicine, Department of Physiology and Croatian Institute for Brain Research, Zagreb, Croatia.
- 557 Hospital for Sick Children, Program in Cell Biology Department, Toronto, Canada; University of Toronto, Department of Biochemistry, Toronto, Canada.
- 558 Chinese Academy of Medical Sciences & Peking Union Medical College, Institute of Medicinal Biotechnology, Beijing, China.
- 559 Virginia Commonwealth University, Department of Biochemistry and Molecular Biology, Richmond, VA, USA.
- 560 Centre for Cancer Biology, University of South Australia, Adelaide, Australia.
- 561 Division of Developmental Biology, National Institute of Child Health & Human Development, National Institutes of Health, Bethesda, MD, USA.
- 562 University of North Carolina at Chapel Hill, Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA.
- 563 University of New Mexico Health Sciences Center, Autophagy, Inflamamtion and Metabolism (AIM) Center, Albuquerque, NM, USA.
- 564 Institut National de la Recherche Scientifique, Centre Armand-Frappier Santé Biotechnologie, Laval, QC, Canada.
- 565 Bellvitge Biomedical Research Institute-IDIBELL, Oncobell Program. L'Hospitalet de Llobregat, Barcelona, Spain.
- 566 University of California, San Diego; Section of Cell and Developmental Biology, La Jolla, CA, USA.
- 567 University of Zurich, Institute of Physiology, Zurich, Switzerland.
- 568 Walter and Eliza Hall Institute of Medical Research, Ubiquitin Signalling Division, Melbourne, Australia; University of Melbourne, Department of Medical Biology, Melbourne, Australia.
- 569 University of Naples "Federico II", Department of Chemical, Materials and Industrial Production Engineering, Naples, Italy.
- 570 University of Perugia, Department of Chemistry, Biology and Biotechnology, Perugia, Italy.
- 571 Sapienza University of Rome, Department of Biochemical Sciences A. Rossi Fanelli, Rome, Italy.
- 572 Philipps University of Marburg, Department of Visceral, Thoracic and Vascular Surgery, Marburg, Germany.
- 573 IRCCS Foundation Ca' Granda Ospedale Maggiore Policlinico, Dino Ferrari Center, Neuroscience Section, University of Milan, Department of Pathophysiology and Transplantation, Milan, Italy.
- 574 University of Teramo, Faculty of Veterinary Medicine, Teramo, Italy.
- 575 Western University, Department of Physiology and Pharmacology, London, ON, Canada.
- 576 Harvard Medical School, Boston Children's Hospital, F.M. Kirby Neurobiology Center, Translational Neuroscience Center, Department of Neurology, Boston, MA, USA.
- 577 National Institute for Infectious Diseases "L. Spallanzani" IRCCS, Rome, Italy.
- 578 National and Kapodistrian University of Athens, Department of Biology, Panepistimioupolis, Athens, Greece.
- 579 University of Cincinnati College of Medicine, Department of Cancer Biology, Cincinnati, OH, USA.
- 580 University of Chile, Faculty of Chemical and Pharmaceutical Sciences, Department of Chemical Pharmacology and Toxicology, Santiago de Chile, Chile.
- 581 University of Alcalá, Deparment of Systems Biology, Biochemistry and Molecular Biology, Alcalá de Henares, Madrid, Spain.
- 582 Arizona State University, Phoenix, AZ, USA.
- 583 Seoul National University, College of Pharmacy, Seoul, Korea.
- 584 University of Montreal, Faculty of Medicine, Microbiology, Infectious diseases and Immunology Department, CRCHUM, Montréal, QC, Canada.
- 585 Goethe University, Institute of Biochemistry II, Faculty of Medicine, Frankfurt am Main, Germany.
- 586 Zhejiang University, School of Medicine, Department of Cell Biology, Hangzhou, Zhejiang Province, China.
- 587 University of Kansas Medical Center, Department of Pharmacology, Toxicology and Therapeutics, Kansas City, KS, USA.
- 588 University of Rome La Sapienza, Department of Biology and Biotechnology C. Darwin, Rome, Italy.
- 589 University of Belgrade, Institute for Biological Research "Sinisa Stankovic" - National Institute of Republic of Serbia, Department of Neurobiology, Belgrade, Serbia.
- 590 University of Belgrade, Institute of Molecular Genetics and Genetic Engineering, Belgrade, Serbia.
- 591 Johns Hopkins University School of Medicine, Department of Pharmacology and Molecular Sciences and Department of Medicine, Baltimore, MD, USA.
- 592 Imperial College London, Department of Life Sciences and MRC Centre for Molecular Bacteriology and Infection, London, UK.
- 593 Friedrich-Alexander University (FAU) Erlangen-Nürnberg and Universitätsklinikum Erlangen, Department of Internal Medicine 3 - Rheumatology and Immunology, Erlangen, Germany.
- 594 Washington University School of Medicine and John Cochran VA Medical Center, Department of Medicine, St. Louis, MO, USA.
- 595 University of Manitoba, Institute of Cardiovascular Sciences, Department of Physiology and Pathophysiology, Winnipeg, Manitoba, Canada.
- 596 Centre de Recherche des Cordeliers, INSERM UMRS 1138, Sorbonne Université, Université de Paris, Equipe 11 labellisée par la Ligue contre le Cancer, 75006 Paris, France; Metabolomics and Cell Biology Platforms, Institut Gustave Roussy, 94805 Villejuif, France.
- 597 University of Calgary, Department of Comparative Biology and Experimental Medicine, Calgary, AB, Canada.
- 598 University of Colima, University Center for Biomedical Research, Colima, Mexico.
- 599 University of Canterbury, Biomolecular Interaction Centre, School of Biological Sciences, Christchurch, New Zealand.
- 600 Hacettepe University, Faculty of Medicine, Department of Medical Biology, Ankara, Turkey.
- 601 University of Kentucky, College of Medicine, Markey Cancer Center, Lexington, KY, USA.
- 602 University of Texas Southwestern Medical Center, Department of Internal Medicine, Center for Autophagy Research, Dallas, TX, USA.
- 603 Jinshan Branch of Shanghai Sixth People's Hospital, Department of Laboratory Medicine, Shanghai, China.
- 604 Washington University in St Louis School of Medicine, Department of Internal Medicine, St. Louis, MO, USA.
- 605 Goethe University, Institute of Biophysical Chemistry, Frankfurt, Germany.
- 606 Nanjing University, Medical School, Division of Immunology, Nanjing, Jiangsu, China.
- 607 Emory University School of Medicine, Department of Psychiatry and Behavioral Sciences, Atlanta, GA, USA.
- 608 Umeå University, Department of Chemistry, Umeå, Sweden.
- 609 University of Arkansas, Center of Excellence for Poultry Science, Fayetteville, AR, USA.
- 610 Oncogenetic Laboratory, Meir Medical Center, Kfar Saba, and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
- 611 Tongren Hospital, Shanghai Jiaotong University School of Medicine, Department of Neurology, Shanghai, China.
- 612 University of British Columbia, Department of Urologic Sciences, Vancouver, BC, Canada.
- 613 National Institute of Biological Sciences, Beijing, China.
- 614 Central South University, The Second Xiangya Hospital, Department of Nephrology, Changsha , Hunan, China.
- 615 Chinese Academy of Medical Sciences and Peking Union Medical College, Institute of Blood Transfusion, Chengdu, China.
- 616 Cardiff University, Division of Cancer and Genetics, Heath Park, Cardiff, UK.
- 617 Centro Andaluz de Biología Molecular y Medicina Regenerativa CABIMER, Consejo Superior de Investigaciones Científicas, Universidad de Sevilla, Universidad Pablo de Olavide, Seville, Spain.
- 618 Shanghai Proton and Heavy Ion Center, Department of Research & Development, Pudong, Shanghai, China.
- 619 University Medical Center Hamburg-Eppendorf, Department of Oncology, Hematology and Bone Marrow Transplantation with Section Pneumology, Hamburg, Germany.
- 620 A.V. Zhirmunsky National Scientific Center of Marine Biology, Laboratory of Pharmacology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russian Federation.
- 621 Far Eastern Federal University, School of Natural Sciences, Vladivostok, Russian Federation.
- 622 Department of Neurology, Boston Children's Hospital, Boston, MA, USA.
- 623 Medical University of Vienna, Department of Dermatology, Division for Biology and Pathobiology of the Skin, Vienna, Austria.
- 624 University of Colorado Anschutz Medical Campus, Division of Renal diseases, Aurora, CO, USA.
- 625 University of Manitoba, Department of Physiology and Pathophysiology, Regenerative Medicine Program, Winnipeg, Manitoba, Canada.
- 626 University of Cologne, Centre for Biochemistry, Institute of Biochemistry I, Medical Faculty, Cologne, Germany.
- 627 United Arab Emirates University, College of Medicine & Health Sciences, Department of Anatomy, Al Ain, UAE.
- 628 University of Graz, NAWI Graz, Institute of Molecular Biosciences, Graz, Austria; BioTechMed Graz, Graz, Austria; University of Graz, Field of Excellence BioHealth, Graz, Austria.
- 629 Long Beach VA and University of California, Irvine, Irvine, CA, USA.
- 630 Ain Shams University, Faculty of Medicine, Department of Medical Biochemistry and Molecular Biology, Cairo, Egypt.
- 631 Hospital Universitari de Tarragona Joan XXIII, Institut d'Investigació Santitària Pere Virgili.
- 632 University of Illinois at Chicago, Department of Pathology, College of Medicine, Chicago, IL, USA.
- 633 Florida International University, Department of Immunology and Nano-Medicine, Miami, FL, USA.
- 634 Children's Cancer Hospital Egypt 57357, Tumor Biology Research Program, Cairo, Egypt.
- 635 University of Camerino, Department of Biosciences and Biotechnology, Camerino, Italy.
- 636 Damietta University, Faculty of Science, Biochemistry Department, Damietta, Egypt.
- 637 Technische Universität Dresden, Medical Clinic I, University Hospital Carl Gustav Carus, Dresden, Germany; Technische Universität Dresden, Faculty of Medicine, Institute for Clinical Chemistry and Laboratory Medicine, Dresden, Germany; Institute of Molecular Genetics of the ASCR, Department of Cancer Cell Biology, Prague, Czech Republic.
- 638 Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (INBIRS), Universidad de Buenos Aires-CONICET, Argentina.
- 639 University of Sheffield, The Bateson Centre, Department of Infection, Immunity and Cardiovascular Disease,Sheffield, South Yorkshire, UK.
- 640 Philipps University of Marburg, Department of Cytobiology and Cytopathology, Marburg, Germany.
- 641 Sanford Burnham Prebys Medical Discovery Research Institute, La Jolla, CA, USA.
- 642 Bogazici University, Department of Molecular Biology and Genetics, Bebek, Istanbul, Turkey.
- 643 Pfizer, Oncology Research & Development, Pearl River, NY, USA.
- 644 Oslo University Hospital, Institute for Cancer Research, Department of Tumor Biology, Montebello, Oslo, Norway.
- 645 University of Bergen, Department of Biomedicine/ Centre for Cancer Biomarkers (CCBIO), Bergen, Norway.
- 646 Instituto de Investigaciones Biomédicas Alberto Sols, C.S.I.C./U.A.M., Madrid, Spain.
- 647 Universität zu Köln, CECAD Forschungszentrum, Institut für Genetik, Köln, Germany.
- 648 University of Turku, Institute of Biomedicine, Turku, Finland.
- 649 IRIM, University of Montpellier, CNRS, Montpellier, France.
- 650 Medical University of Lodz, Department of Laboratory Diagnostics, Lodz, Poland.
- 651 Sapienza University of Rome, DAHFMO-Section of Anatomy, Rome, Italy.
- 652 Istituto Superiore di Sanità, Department of Oncology and Molecular Medicine, Rome, Italy.
- 653 Karolinska Institutet, Institute of Environmental Medicine, Stockholm, Sweden.
- 654 Universidad Nacional de Cuyo, Facultad de Ciencias Médicas, Laboratorio de Biología Celular y Molecular, IHEM-CONICET, Mendoza, Argentina.
- 655 Max-Planck-Institute of Biophysical Chemistry, Biochemistry of Signal Dynamics, Göttingen, Germany.
- 656 La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria, Australia; Olivia Newton-John Cancer Research Institute, Heidelberg, Victoria, Australia; School of Cancer Medicine, La Trobe University, Melbourne, Victoria, Australia.
- 657 Universidad Miguel Hernández (UMH), Instituto de Investigación, Desarrollo e Innovación en Biotecnología Sanitaria de Elche (IDiBE), Elche, Spain.
- 658 Dresden University Medical Center, Department of Neurology, Dresden, Germany.
- 659 University of South Carolina School of Medicine, Department of Cell Biology and Anatomy, Columbia, SC, USA.
- 660 University of Pittsburgh, Department of Surgery, Pittsburgh, PA, USA.
- 661 University of Cincinnati, Department of Cancer Biology, Cincinnati, OH, USA.
- 662 University of Oslo and Akershus University Hospital, Department of Clinical Molecular Biology, Lørenskog, Norway; The Norwegian Centre on Healthy Ageing (NO-Age), Oslo, Norway.
- 663 Shanghai Institute of Organic Chemistry, Chinese Academy of Science, Interdisciplinary Research Center on Biology and Chemistry, Shanghai, China; University of Chinese Academy of Sciences, Beijing, China.
- 664 The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China.
- 665 King's College London, Department of Basic and Clinical Neuroscience, London, United Kingdom; Institut du Cerveau et de la Moelle épinière (ICM), Paris, France.
- 666 Centre International de Recherche en Infectiologie (CIRI), University Lyon, Inserm, U1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, ENS Lyon, Lyon, France.
- 667 Rudolf Virchow Center, University of Würzburg, Würzburg, Germany.
- 668 South Australian Health and Medical Research Institute, Hopwood Centre for Neurobiology, Lifelong Health Theme, Adelaide, South Australia, Australia.
- 669 Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA.
- 670 Guangzhou Medical University, School of Basic Medical Sciences, State Key Laboratory of Respiratory Disease, Guangzhou, China.
- 671 The First Hospital of Jilin University, Department of Neurology, Changchun, China.
- 672 Medical School of Zhejiang University, Sir Run Run Shaw Hospital, Key lab of Biotherapy in Zhejiang, Laboratory of Cancer Biology, Hangzhou, China.
- 673 The University of Hong Kong, School of Chinese Medicine, Li Ka Shing Faculty of Medicine, Hong Kong, China.
- 674 Baylor College of Medicine, Department of Biochemistry and Molecular Biology, Houston TX, USA.
- 675 Chinese Academy of Sciences, Institute of Biophysics, Beijing, China.
- 676 University of São Paulo (USP), Ribeirão Preto Medical School, Department of Genetics, Ribeirão Preto, SP, Brazil.
- 677 Washington University in St. Louis, Department of Ophthalmology and Visual Sciences, St. Louis, MO, USA.
- 678 University of Texas Southwestern Medical Center, Center for Autophagy Research, Department of Internal Medicine and Howard Hughes Medical Institute, Dallas, TX, USA.
- 679 Institute of Biomedical Research of Barcelona (IIBB)-CSIC, Barcelona, Spain; Liver Unit, Hospital Clinic i Provincial de Barcelona, IDIBAPS and CIBEREHD, Barcelona, Spain; Research Center for ALPD, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
- 680 Columbia University, New York, NY, USA.
- 681 Università del Piemonte Orientale, Department of Health Sciences, Laboratory of Molecular Pathology, Novara, Italy.
- 682 University of Sao Paulo, Institute of Biosciences, Sao Paulo, SP, Brazil.
- 683 University of California, San Diego, Department of Cellular and Molecular Medicine, La Jolla, CA, USA.
- 684 Instituto de Investigaciones Marinas CSIC, Department of Biotechnology and Aquaculture, Immunology and Genomics, Vigo, Spain.
- 685 University of Padua, Department of Biomedical Sciences, Padua, Italy; Italian National Research Council (CNR), Neuroscience Institute, Padua, Italy.
- 686 University of Piemonte Orientale, Department of Translational Medicine, Novara, Italy.
- 687 Universidade Federal do Rio Grande do Sul (UFRGS), Morphological Sciences Department, Porto Alegre, RS, Brazil; Clinicas Hospital of Porto Alegre, Porto Alegre, RS, Brazil.
- 688 Danish Cancer Society Research Center, Redox Signaling and Oxidative Stress Group, Copenhagen, Denmark.
- 689 Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy.
- 690 Università degli Studi Sapienza di Roma, SAIMLAL Department, Roma, Italy.
- 691 Center for Systems and Therapeutics, Gladstone Institutes; Departments of Neurology and Physiology, University of California San Francisco, San Francisco, CA, USA.
- 692 NYU School of Medicine, Department of Medicine, New York, NY, USA.
- 693 Virginia Commonwealth University, School of Medicine, Department of Human and Molecular Genetics, Richmond, VA, USA; Virginia Commonwealth University, VCU Institute of Molecular Medicine, Richmond, VA, USA; Columbia University, College of Physicians and Surgeons, Departments of Pathology, Neurosurgery and Urology, New York, NY, USA.
- 694 State University of New York, University at Buffalo, Jacobs School of Medicine and Biomedical Sciences, Departments of Ophthalmology/Biochemistry and Neuroscience Program, Buffalo, NY, USA.
- 695 Norwegian University of Science and Technology, Department of Clinical and Molecular Medicine, Centre for Molecular Inflammation Research, Trondheim, Norway.
- 696 Central University of Tamil Nadu, Thiruvarur, Tamil Nadu, India.
- 697 Babraham Institute, Signalling ISP, Cambridge, UK.
- 698 University of Genova, Department of Internal Medicine and IRCCS Policlinico San Martino, Genova, Italy.
- 699 Montreal Neurological Institute, Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada.
- 700 University of Pavia, Department of Molecular Medicine, Biochemistry Unit, Pavia, Italy.
- 701 University of Pisa, Department of Translational Medicine and New Technologies in Medicine and Surgery, Pisa, Italy; I.R.C.C.S. Neuromed Pozzilli, Pozzilli, Italy.
- 702 Istituto Superiore di Sanità, Department of Environment and Health, Section of Mechanisms, Biomarkers and Models, Rome, Italy.
- 703 University of Texas Medical Branch, Mitchell Center for Neurodegenerative Diseases, Department of Neurology, Galveston, TX, USA.
- 704 University of Nebraska-Lincoln, Redox Biology Center, Lincoln, NE, USA.
- 705 Laboratory of Sex-gender Medicine, National Institute of Biostructures and Biosystems, Sassari, Italy.
- 706 Icahn School of Medicine at Mount Sinai, Department of Medicine, Division of Liver Diseases, New York, NY, USA.
- 707 Institute of Science and Technology Austria (IST), Klosterneuburg, Austria.
- 708 Clínica Universidad de Navarra, Metabolic Research Laboratory, CIBEROBN, IdiSNA, Pamplona, Spain.
- 709 University of Extremadura, Centro de Investigación Biomedica en Red de Enfermedades Neurodegenerativas (CIBERNED), Instituto de Investigación Biosanitaria de Extremadura (INUBE), Department of Biochemistry, Molecular Biology and Genetics, Faculty of Nursing and Occupational Therapy, Cáceres, Spain.
- 710 Kyushu University, Medical Institute of Bioregulation, Fukuoka, Japan.
- 711 Tokyo Institute of Technology, Institute of Innovative Research, Cell Biology Center, Yokohama, Japan.
- 712 National Institute of Neuroscience, National Center of Neurology and Psychiatry, Department of Degenerative Neurological Diseases, Kodaira, Tokyo, Japan.
- 713 Tohoku University, Graduate School of Life Sciences, Department of Integrative Life Sciences, Sendai, Miyagi, Japan.
- 714 Goethe-University Frankfurt, Institute for Experimental Cancer Research in Pediatrics, Frankfurt, Germany.
- 715 Monash University, Cancer Program, Biomedicine Discovery Institute and Department of Anatomy & Developmental Biology, VIC, Australia; Peter MacCallum Cancer Centre, Prostate Cancer Translational Research Laboratory, Melbourne, Victoria, Australia; University of Melbourne, Sir Peter MacCallum Department of Oncology, Parkville, VIC, Australia.
- 716 Juntendo University Graduate School of Medicine, Department of Neurology, Bunkyo-ku, Tokyo Japan.
- 717 The University of Chicago, Department of Microbiology, Chicago, IL, USA.
- 718 University of Gdansk, Department of Molecular Biology, Gdansk, Poland.
- 719 New York University - Abu Dhabi, Saadiyat Island Campus, Division of Science (Biology), Cell Death Signaling Laboratory, Abu Dhabi, UAE.
- 720 University of Oxford, Sir William Dunn School of Pathology, Oxford, UK.
- 721 Universidad Castilla La Mancha, Facultad de Farmacia, Área Tecnología Farmacéutica, Albacete, Spain.
- 722 Beckman Research Institute at City of Hope, Department of Systems Biology, Monrovia, CA, USA.
- 723 Weill Cornell Medical College, Department of Radiation Oncology, New York, NY, USA; Sandra and Edward Meyer Cancer Center, New York, NY, USA; Caryl and Israel Englander Institute for Precision Medicine, New York, NY, USA; Yale School of Medicine, Department of Dermatology, New Haven, CT, USA; Université de Paris, Paris, France.
- 724 Sorbonne Université, Developmental Biology Laboratory, CNRS, Institut de Biologie Paris Seine, IBPS, UMR7622, Paris, France.
- 725 University of Edinburgh, Cancer Research UK Edinburgh Centre, Institute of Genetics and Molecular Medicine, Edinburgh, UK.
- 726 University of Texas MD Anderson Cancer Center, Department of Experimental Radiation Oncology, Houston, TX, USA.
- 727 MRC Protein Phosphorylation and Ubiquitylation Unit, School of Life Sciences, University of Dundee, UK.
- 728 Fourth Military Medical University, School of Aerospace Medicine, Xi'an, China.
- 729 Chinese Academy of Sciences, Institute of Zoology, State Key Laboratory of Stem cell and Reproductive Biology, Beijing, China.
- 730 Harbin Institute of Technology, the HIT Center for Life Sciences, School of Life Sciences and Technology, Harbin, China.
- 731 South China University of Technology, School of Medicine and Institute for Life Sciences, GuangZhou, China.
- 732 University of Pittsburgh School of Medicine, Hillman Cancer Center and Department of Microbiology and Molecular Genetics, Pittsburgh, PA, USA.
- 733 University of Pittsburgh School of Medicine, Department of Surgery, Pittsburgh, PA, USA.
- 734 Chinese Academy of Sciences Center for Excellence in Molecular and Cellular Sciences, Shanghai Institute of Biochemistry and Cell Biology, Shanghai, China.
- 735 Universitat de Lleida, Department of Experimental Medicine, IRBLleida, Lleida, Spain.
- 736 Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Departamento de Microbiología, Inmunología y Biotecnología, Cátedra de Inmunología, Buenos Aires, Argentina; Universidad de Buenos Aires, CONICET, Instituto de Estudios de la Inmunidad Humoral (IDEHU), Buenos Aires, Argentina.
- 737 Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Instituto de Química Biológica de la Facultad de Ciencias Exactas y Naturales (IQUIBICEN), Buenos Aires, Argentina.
- 738 National Center for Biotechnology (CNB)-CSIC, Laboratory of Intracellular Bacterial Pathogens, Madrid, Spain.
- 739 Universidad Autónoma de Madrid, Facultad de Medicina, Departamento de Anatomía, Histología y Neurociencia, Madrid, Spain.
- 740 Universidad Autonoma de Nuevo Leon, School of Medicine, Department of Histology, Monterrey, Nuevo Leon, Mexico.
- 741 Universidad de Salamanca, Institute of Functional Biology and Genomics (IBFG), Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, Spain.
- 742 Department of Cell Biology and Histology, Faculty of Biology, University of Murcia, IMIB-Arrixaca, Murcia, Spain.
- 743 Department of Cell Death and Proliferation, Institute of Biomedical Research of Barcelona (IIBB- CSIC); Liver Unit, Hospital Clinic i Provincial de Barcelona, IDIBAPS, CIBEREHD, Barcelona, Spain.
- 744 Cajal Institute/CSIC and CIBERNED, ISCIII, Madrid, Spain.
- 745 KU Leuven, Department of Cellular and Molecular Medicine, Leuven, Belgium.
- 746 Centro de Biología Molecular Severo Ochoa (CSIC-UAM), Madrid, Spain.
- 747 Sapienza University of Rome, Department of Experimental Medicine, Rome, Italy.
- 748 Tulane University, Department of Microbiology and Immunology, New Orleans, LA, USA.
- 749 University of Bonn, Clinical Centre, Department of Psychiatry, Neurohomeostasis Group, Bonn, Germany.
- 750 Zhejiang University School of Medicine,Women's Hospital,Hangzhou, Zhejiang, China.
- 751 University of Milano, Department of Biomedical Sciences for Health, Milan, Italy.
- 752 Institut de Biologie Moléculaire des Plantes, Centre National de la Recherche Scientifique, Unité Propre de Recherche 2357, Conventionné avec l'Université de Strasbourg, Strasbourg, France.
- 753 University Clinic Freiburg, Institute for Microbiology and Hygiene, University of Freiburg - Faculty of Medicine, Freiburg, Germany.
- 754 Mortimer B. Zuckerman Mind Brain and Behavior Institute, Columbia University, New York, NY, USA.
- 755 Institute for Animal Developmental and Molecular Biology, Heinrich-Heine University Düsseldorf, Düsseldorf, Germany.
- 756 Virginia Commonwealth University, Departments of Pharmacology, Toxicology and Medicine and Massey Cancer Center, Richmond, VA, USA.
- 757 Kerman University of Medical Sciences, Institute of Neuropharmacology, Neuroscience Research Center, Kerman, Iran.
- 758 Autophagy Research Center, Health Policy Research Center, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran.
- 759 University of Torino, Department of Molecular Biotechnology and Health Sciences, Torino, Italy.
- 760 KU Leuven, Department of Public Health and Primary Care, Centre Environment & Health, Leuven, Belgium.
- 761 University of Sussex, School of Life Sciences, Department of Biochemistry and Biomedicine, Brighton, UK.
- 762 Sapienza University of Rome, Department of Anatomy, Histology, Forensic Medicine and Orthopedics, Rome, Italy.
- 763 Democritus University of Thrace, Medical School, Department of Pathology, Alexandroupolis, Greece.
- 764 Weill Cornell Medicine, Brain and Mind Research Institute, Burke Neurological Institute, White Plains, NY, USA.
- 765 University of Manitoba, Departments of Biochemistry and Medical Genetics, Winnipeg, Canada.
- 766 Department of Fundamental Neurosciences, University of Lausanne, Lausanne, Switzerland.
- 767 Clinic of Neonatology, Department of Women, Mother and Child, University Hospital Center of Vaud (CHUV), Lausanne, Switzerland.
- 768 National Institutes of Health, National Institutes of Neurological Disorders and Stroke (NINDS), Bethesda, MD, USA.
- 769 University of Ferrara, Department of Morphology, Surgery and Experimental Medicine, Ferrara, Italy.
- 770 University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Faculty of Medicine, Coimbra, Portugal; University of Coimbra, Center for Innovative Biomedicine and Biotechnology, Coimbra, Portugal.
- 771 University of Toronto, Department of Laboratory Medicine and Pathobiology, Toronto, Ontario, Canada.
- 772 University of California, Davis and MIND Institute, School of Veterinary Medicine, Dept. Molecular Biosciences, Davis, CA, USA.
- 773 INSERM, UMR1037 CRCT, Toulouse, France; Université Toulouse III-Paul Sabatier, UMR1037 CRCT, Toulouse, France; CNRS, ERL5294 CRCT, Toulouse, France.
- 774 University of Cologne, Faculty of Medicine and University Hospital Cologne, Institute for Medical Microbiology, Immunology and Hygiene, Cologne, Germany.
- 775 University of Seville, Department of Genetics, Seville, Spain.
- 776 Ludwig Institute for Cancer Research; University of California San Diego, Department of Cellular and Molecular Medicine, La Jolla, CA, USA.
- 777 Medical University of Warsaw, Department of Immunology, Warsaw, Poland.
- 778 University of Auckland, School of Biological Sciences, Auckland, New Zealand.
- 779 Luxembourg Institute of Health, Department of Oncology, NorLux Neuro-Oncology Laboratory, Luxembourg, Luxembourg.
- 780 Center of Toxins, Immune-response and Cell Signaling (CeTICS) and Laboratório Especial de Ciclo Celular (LECC), Instituto Butantan, São Paulo, Brazil.
- 781 University of Groningen, University Medical Center of Groningen, Department of Biomedical Sciences of Cells and Systems, Groningen, The Netherlands.
- 782 Leiden University Medical Center, Department of Cell and Chemical Biology and Oncode Institute, Leiden, The Netherlands.
- 783 Shanghai Jiao Tong University, School of Life Sciences and Biotechnology, Shanghai, China.
- 784 University of León, Institute of Biomedicine (IBIOMED) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), León, Spain.
- 785 University of La Laguna, Institute of Biomedical Technologies, Department of Basic Medical Sciences, La Laguna, Tenerife, Spain.
- 786 University of Alberta, Department of Biochemistry, Edmonton, Alberta, Canada.
- 787 University of Alabama at Birmingham, Department of Optometry and Vision Science, Birmingham, AL, USA.
- 788 The Henry M. Jackson Foundation, Inc. Bethesda, MD, USA.
- 789 Technische Universität München, Klinikum rechts der Isar, Comprehensive Cancer Center München, München, Germany.
- 790 Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Instituto de Química Biológica Ciencias Exactas y Naturales (IQUIBICEN), Facultad de Ciencias Exactas y Naturales, Departamento de Química Biológica, Laboratorio de Disfunción Celular en Enfermedades Neurodegenerativas y Nanomedicina, Buenos Aires, Argentina.
- 791 BC Cancer, Michael Smith Genome Sciences Centre, Vancouver, BC, Canada.
- 792 Harvard Medical School, Department of Dermatology, Cutaneous Biology Research Center Massachusetts General Hospital, Boston, MA, USA.
- 793 Consejo Superior de Investigaciones Científicas, Instituto de Bioquímica Vegetal y Fotosíntesis (CSIC-IBVF), Sevilla, Spain.
- 794 Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
- 795 Tel Aviv University, Sackler Faculty of Medicine and Sagol School of Neuroscience, Department of Human Molecular Genetics and Biochemistry, Tel Aviv, Israel.
- 796 Max Planck Institute for Biology of Ageing, Cologne, Germany; University of Cologne, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany.
- 797 Jena University Hospital, Department of Anesthesiology and Intensive Care Medicine, Center for Molecular Biomedicine (CMB) and Center for Sepsis Control and Care (CSCC), Jena, Germany.
- 798 Weill Cornell Medicine, Feil Family Brain and Mind Research Institute, New York, NY, USA.
- 799 University of Texas MD Anderson Cancer Center, Department of Cancer Systems Imaging, Houston, TX, USA.
- 800 University of the West of England, Department of Applied Sciences, Bristol, UK.
- 801 Flinders University, College of Medicine and Public Health, Adelaide, Australia.
- 802 University of Alabama at Birmingham,Center for Neurodegeneration and Experimental Therapeutics, Department of Neurology, Birmingham, AL, USA.
- 803 Boston University, Departments of Biomedical Engineering, Chemistry, and Medicine, Boston, MA, USA.
- 804 Université de Strasbourg/CNRS UPR 3572 Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France.
- 805 University of Iowa, Departments of Pediatrics and Microbiology, Iowa City, IA, USA.
- 806 Max Planck Institute for Biology of Ageing, Cologne, Germany.
- 807 German Institute of Human Nutrition, Department of Molecular Toxicology, Nuthetal, Germany.
- 808 Lanzhou University, School of Public Health, Lanzhou, Gansu, China.
- 809 Case Western Reserve University, Comprehensive Cancer Center, Cleveland, OH, USA.
- 810 University of Salento, Department of Biological and Environmental Sciences and Technologies, Lecce, Italy.
- 811 Research Center Principe Felipe, Cellular Pathology Laboratory, Valencia, Spain.
- 812 Johns Hopkins University School of Medicine, Department of Neuroscience, Baltimore, MD, USA.
- 813 School of Pharmacy, Complutense University, Madrid, Spain; Centro de Investigación Biomédica en Red (CIBER) de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Madrid, Spain.
- 814 Dalhousie University, Faculty of Medicine, Departments of Pathology, Biology, and Microbiology & Immunology, Halifax, NS, Canada.
- 815 David Geffen School of Medicine at UCLA, Department of Medicine, Los Angeles, CA, USA.
- 816 KU Leuven, Clinical Division and Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, Leuven, Belgium.
- 817 Justus-Liebig University, Department of Internal Medicine, Giessen, Germany.
- 818 Center for Molecular Medicine, Maine Medical Center Research Institute, Scarborough, ME, USA.
- 819 Tongji University School of Medicine, Shanghai Tenth People's Hospital, Department of Gastroenterology, Shanghai, China.
- 820 The University of Sheffield, Department of Biomedical Science, Firth Court, Western Bank, Sheffield, UK.
- 821 University of Virginia, School of Medicine, Department of Surgery, Charlottesville, VA, USA.
- 822 University of California, Los Angeles, Department of Neurology, Molecular and Medical Pharmacology, David Geffen School of Medicine, Los Angeles, CA, USA.
- 823 Institute of Microbial Technology, Department of Molecular Biology, Chandigarh, India.
- 824 CSIR-Central Drug Research Institute, Lucknow, India.
- 825 University of Oslo, Institute of Basic Medical Sciences, Oslo, Norway.
- 826 Deakin University, School of Medicine, Faculty of Health, Victoria, Australia.
- 827 Macquarie University, Faculty of Medicine, Health and Human Sciences, New South Wales, Australia.
- 828 University of California, San Diego, Skaggs School of Pharmacy and Pharmaceutical Sciences, La Jolla, CA, USA.
- 829 Medical College of Wisconsin, Department of Medicine and Cardiovascular Center, Milwaukee, WI, USA.
- 830 ICAR-Indian Veterinary Research Institute, FMD VP Laboratory, Bengaluru, India.
- 831 University of Eastern Finland, A.I. Virtanen Institute for Molecular Sciences, Kuopio, Finland.
- 832 Brandeis University, Department of Biology and Rosenstiel Basic Medical Sciences Research Center, Waltham, MA, USA.
- 833 University of Gdansk, Department of Medical Biology and Genetics, Gdansk, Poland.
- 834 Tokai University School of Medicine, Department of Molecular Life Sciences, Isehara, Kanagawa, Japan.
- 835 Swedish Agricultural University (SLU), Department of Plant Biology, Uppsala, Sweden.
- 836 Hasselt University, Biomedical Research Institute, Diepenbeek, Belgium.
- 837 University of Surrey, School of Bioscience and Medicine, Department of Microbial Sciences, Stag Hill Campus, Guildford, Surrey, UK.
- 838 Barts Cancer Institute, Queen Mary University of London, Centre for Biotherapeutics and Biomarkers, London, UK.
- 839 Johns Hopkins University Bloomberg School of Public Health, W. Harry Feinstone Department of Molecular Microbiology and Immunology, Baltimore, MD, USA.
- 840 Goethe University, Institute for Molecular Biosciences, Frankfurt/Main, Germany.
- 841 Osaka University, Graduate School of Medicine, Department of Genetics, Suita, Osaka, Japan.
- 842 Zhejiang University, Sir Run Run Shaw Hospital, College of Medicine, Department of Medical Oncology, Zhejiang, Hangzhou, China.
- 843 Sanford Burnham Prebys Medical Discovery Institute, Program of Development, Aging and Regeneration, La Jolla, CA, USA.
- 844 University of Michigan, Department of Biological Chemistry, Ann Arbor, MI, USA.
- 845 Third Department of Internal Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
- 846 Department of Neurophysiology, Institute for Biological Research "Siniša Stanković" - National Institute of Republic of Serbia, University of Belgrade, Belgrade, Serbia.
- 847 University of California, Davis, School of Medicine, Department of Pharmacology, Davis, CA, USA.
- 848 University of Toronto, Department of Medicine, Toronto, Ontario, Canada.
- 849 Monash University, School of Clinical Sciences at Monash Health, Centre for Inflammatory Diseases, Rheumatology Group, Clayton, Victoria, Australia.
- 850 Tohoku University Graduate School of Medicine, Division of Neurology, Department of Neuroscience & Sensory Organs, Sendai, Japan.
- 851 University of Malaya, Institute of Biological Sciences (Genetics), Kuala Lumpur, Malaysia.
- 852 Washington University School of Medicine, Division of Pulmonary and Critical Care Medicine, St. Louis, MO, USA.
- 853 Leibniz Forschungsinstitut für Molekulare Pharmakologie (FMP), Department of Molecular Pharmacology and Cell Biology, Berlin, Germany.
- 854 California Institute of Technology, Division of Biology and Biological Engineering, Pasadena, CA, USA.
- 855 University of Massachusetts Medical School, Molecular, Cell and Cancer Biology, Worcester, MA, USA.
- 856 Bulgarian Academy of Sciences, Institute of Biology and Immunology of Reproduction Acad. Kiril Bratanov, Sofia, Bulgaria.
- 857 University of Minnesota, Department of Genetics, Cell Biology and Development, Minneapolis, MN, USA.
- 858 Northwestern University, Feinberg School of Medicine, Department of Cell and Developmental Biology, Chicago, IL, USA.
- 859 Sichuan University, West China School of Pharmacy, Key Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Chengdu, China.
- 860 Jinan University, College of Pharmacy, Guangzhou, China.
- 861 Department of Immunology, Duke University Medical Center, Durham, NC, USA.
- 862 University of Chicago, Department of Medicine, Section of Dermatology, Chicago, IL, USA.
- 863 D'Youville College, School of Pharmacy, Department of Pharmaceutical, Social and Administrative Sciences, Buffalo, NY, USA.
- 864 University of Innsbruck, Institute of Biochemistry and Center for Molecular Biosciences Innsbruck, Innsbruck, Austria; University Medical Center Groningen, University of Groningen, Section Systems Medicine of Metabolism and Signaling, Laboratory of Pediatrics, Groningen, The Netherlands.
- 865 National Institutes of Health, National Eye Institute, Ophthalmic Genetics and Visual Function Branch, Ophthalmic Molecular Genetics Section, Bethesda, MD, USA.
- 866 University of Glasgow, Wolfson Wohl Cancer Research Centre, Institute of Cancer Sciences, Glasgow, UK.
- 867 University College London, Cancer Institute, London, UK.
- 868 Universidade Federal de São Carlos, Department of Genetics and Evolution, São Carlos (SP), Brazil.
- 869 Instituto de Investigación Sanitaria de Navarra (IdISNA), Fundación Miguel Servet, Biomedical Research Center of Navarre-Navarrabiomed, Immunomodulation Group, Pamplona, Spain.
- 870 Université de Bordeaux, Institut des Maladies Neurodégénératives (IMN), CNRS UMR 5293, Bordeaux, France.
- 871 University of Barcelona, Barcelona Hepatic Hemodynamic Unit, Liver Unit, Hospital Clinic, Hospital Clínic-Institut d'Investigacions Biomèdiques (IDIBAPS), Centro de Investigación Biomédica Red de enfermedades Hepáticas y Digestivas (CIBERehd), Barcelona, Spain.
- 872 INRA, UR1037 Laboratory of Fish Physiology and Genomics, Campus de Beaulieu, Rennes, France; Hunan Normal University, College of Life Sciences, State Key Laboratory of Developmental Biology of Freshwater Fish, Changsha, Hunan, China.
- 873 University of Lleida, Department of Basic Medical Sciences, IRBLleida, Lleida, Spain.
- 874 Universidad del País Vasco, Instituto Biofisika (CSIC, UPV/EHU) and Departamento de Bioquímica y Biología Molecular, Bilbao, Spain.
- 875 Centenary Institute, The University of Sydney, Newtown, New South Wales, Australia; Faculty of Medicine and Health, The University of Sydney, Newtown, New South Wales, Australia.
- 876 University of Chile, Biomedical Neuroscience Institute, Faculty of Medicine, Santiago, Chile; FONDAP Center for Geroscience Brain Health and Metabolism, Santiago, Chile; University of Chile, Institute of Biomedical Science, Program of Cellular and Molecular Biology, Santiago, Chile; Buck Institute for Research on Aging, Novato, CA, USA.
- 877 University of Bern, Institute of Cell Biology, Bern, Switzerland.
- 878 Kyushu University, Depatment of Bioscience and Biotechnology, Nishi-ku, Fukuoka, Japan.
- 879 UT Southwestern Medical Center, Departments of Medicine (Cardiology) and Molecular Biology, Dallas, TX, USA.
- 880 Theoretical Division, Los Alamos National Laboratory, Los Alamos, NM, USA.
- 881 University of Waterloo, School of Pharmacy, Kitchener, ON, Canada.
- 882 University of Hong Kong, Department of Medicine, Division of Neurology, Pokfulam, Hong Kong, China.
- 883 National University of Singapore, Department of Physiology, Singapore.
- 884 University of North Carolina at Chapel Hill, Department of Pharmacology, Chapel Hill, NC, USA.
- 885 Yale University, Department of Molecular Biophysics and Biochemistry, New Haven, CT, USA.
- 886 Swedish University of Agricultural Sciences (SLU) and Linnean Center for Plant Biology, Uppsala BioCenter, Department of Plant Biology, Uppsala, Sweden.
- 887 University Côte d'Azur, FHU OncoAge, Department of Pathology, Nice, France.
- 888 German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Department of Molecular Toxicology, Nuthetal, Germany.
- 889 University of Helsinki, Medicum, Biochemistry and Developmental Biology, Helsinki, Finland.
- 890 University of Birmingham, College of Medical and Dental Sciences, Institute of Inflammation and Ageing, Birmingham, UK.
- 891 Kyungpook National University, School of Medicine, Department of Physiology, Daegu, Korea.
- 892 Kaohsiung Medical University, Graduate Institute of Medicine & Department of Biochemistry, Faculty of Medicine, College of Medicine, Kaohsiung, Taiwan.
- 893 University of Cologne, Institute for Genetics and CECAD Research Center, Cologne, Germany.
- 894 European Molecular Biology Laboratory, Heidelberg, Germany.
- 895 University of Texas Southwestern Medical Center, Department of Internal Medicine, Dallas, TX, USA.
- 896 Chung Shan Medical University, Institute of Medicine, Taiwan.
- 897 National Chiao-Tung University, Department of Applied Chemistry and Institute of Molecular Science, Hsinchu, Taiwan.
- 898 University of Plymouth, Faculty of Health: Medicine, Dentistry and Human Sciences, Peninsula Dental School, Plymouth, Devon, UK.
- 899 Anhui University of Science and Technology, Department of Medical Immunology, Huainan, China.
- 900 Soochow University, Institute of Neuroscience, Suzhou, Jiangsu Province, China.
- 901 University of Texas Southwestern Medical Center, Department of Internal Medicine, Charles and Jane Center for Mineral Metabolism and Clinical Research, Dallas, TX, USA.
- 902 Southern Medical University, Shenzhen Hospital, Department of Gastroenterology, Shenzhen, Guangdong.
- 903 National Tsing Hua University, Department of Chemical Engineering, and Frontier Research Center on Fundamental and Applied Sciences of Matters, Hsinchu, Taiwan.
- 904 Northwest A&F University, College of Veterinary Medicine, Shaanxi Centre of Stem Cells Engineering & Technology, Yangling, Shaanxi, China.
- 905 Shanghai Jiao Tong University School of Medicine, Shanghai General Hospital, Department of Orthopedics, Shanghai, China; Shanghai Bone Tumor Institution, Shanghai, China.
- 906 State University of New York, Downstate Health Sciences University, Department of Surgery and Cell Biology, Brooklyn, NY, USA.
- 907 Sichuan University, and Collaborative Innovation Center for Biotherapy, West China Hospital, West China School of Basic Medical Sciences & Forensic Medicine, and State Key Laboratory of Biotherapy and Cancer Center, Chengdu, China.
- 908 New York University School of Medicine, Department of Environmental Medicine, Urology, Biochemistry and Molecular Pharmacology, New York, NY, USA.
- 909 Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Immunology & Department of Immunology and Microbiology, Shanghai, China.
- 910 University of Sydney, Kolling Institute, Renal Research Lab, Sydney, New South Wales, Australia.
- 911 Wenzhou Medical University, School of Laboratory Medicine and Life Sciences, Wenzhou, Zhejiang Province, China.
- 912 Huazhong University of Science and Technology, Tongji Medical College, School of Pharmacy, Wuhan, Hubei, China.
- 913 Chongqing University, School of Medicine, Center for Neurointelligence, Chongqing, China.
- 914 The Second Affiliated Hospital of Xi'an Jiaotong University, Department of Radiation Oncology, Xi'an, Shaan Xi, China.
- 915 Zhejiang University School of Medicine, The First Affiliated Hospital, Zhejiang Provincial Key Laboratory of Pancreatic Disease, Hangzhou, Zhejiang, China.
- 916 III. Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
- 917 Medical University of Vienna, Department of Pathology, Vienna, Austria.
- 918 University Hospital Jena, Institute of Human Genetics, Jena, Thuringia, Germany.
- 919 University of Otago, School of Biomedical Sciences and Brain Health Research Centre, Department of Biochemistry, Dunedin, New Zealand.
- 920 University of Bern, Institute of Pathology, Bern, Switzerland; TRANSAUTOPHAGY: European network for multidisciplinary research and translation of autophagy knowledge, COST Action CA15138.
- 921 Max Planck Institute of Biophysics, Department of Theoretical Biophysics, Frankfurt am Main, Germany.
- 922 University of California, Berkeley, Department of Molecular and Cell Biology, Berkeley, CA, USA.
- 923 West Virginia University, School of Medicine, Department of Physiology and Pharmacology, Morgantown, WV, USA.
- 924 Durham University, Department of Biosciences, Durham, UK.
- 925 University of Indonesia, Faculty of Medicine, Department of Obstetric and Gynecology, Jakarta, Indonesia.
- 926 Yonsei University, Department of Biotechnology, Seodaemun-gu, Seoul, Korea.
- 927 The University of Chicago, Department of Pathology, Chicago, IL, USA; present address: VIR Biotechnology, San Francisco, CA, USA.
- 928 University of Messina, Department of Human Pathology in Adult and Developmental Age "Gaetano Barresi", Section of Pathology, Messina, Italy.
- 929 Kyushu University, Medical Institute of Bioregulation (MIB), Fukuoka, Japan.
- 930 Osaka International Cancer Institute, Department of Molecular and Cellular Biology, Osaka, Japan.
- 931 Juntendo University Graduate School of Medicine, Department of Research for Parkinson's Disease, Tokyo, Japan.
- 932 University of Modena and Reggio Emilia, Department of Life Sciences, Modena, Italy.
- 933 Juntendo University, Juntendo University Graduate School of Medicine, Division for Development of Autophagy Modulating Drugs, Tokyo, Japan.
- 934 University of North Texas Health Science Center, North Texas Eye Research Institute and Department of Pharmaceutical Sciences, Fort Worth, TX, USA.
- 935 University of Michigan, Life Sciences Institute, Department of Molecular and Integrative Physiology, Ann Arbor, MI, USA.
- 936 Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR 7258, Aix-Marseille Université and Institut Paoli-Calmettes, Parc Scientifique et Technologique de Luminy, Marseille, France.
- 937 University of Massachusetts Medical School, Department of Microbiology and Physiological Systems, Worcester, MA, USA.
- 938 National University of Córdoba, Clinical Biochemistry Department, CIBICI-CONICET, Córdoba, Argentina.
- 939 Stanford University School of Medicine, Department of Pathology, Stanford, CA, USA.
- 940 Akita University Graduate School of Medicine, Department of Ophthalmology, Akita, Japan.
- 941 Vascular Biology Center, Medical College of Georgia, Augusta University, Augusta, GA, USA; South China University of Technology, Guangzhou First People's Hospital, School of Medicine and Institutes for Life Sciences, Guangzhou, Guangdong, China.
- 942 University of Copenhagen, Copenhagen Biocentre, Faculty of Health and Medical Sciences, Biotech Research & Innovation Centre (BRIC), Neuroinflammation Unit, Copenhagen N, Denmark.
- 943 Chiba University, Department of Biology, Chiba, Japan.
- 944 Keio University School of Medicine, Department of Neurology, Tokyo, Japan.
- 945 University College London, Department of Neuroscience, Physiology and Pharmacology, London, UK.
- 946 Institute for Research in Biomedicine (IRB Barcelona), Barcelona, Spain; Universitat de Barcelona, Departament de Bioquímica I Biomedicina Molecular, Barcelona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
- 947 Barcelona Institute of Science and Technology (BIST), Institute for Research in Biomedicine (IRB Barcelona), Barcelona, Spain; CIBER de Diabetes y Enfermedades Metabólicas Asociadas, Barcelona, Spain; Universitat de Barcelona, Facultat de Biologia, Departament de Bioquimica i Biomedicina Molecular, Barcelona, Spain.
- 948 Hampton University, School of Pharmacy, Department of Pharmaceutical Sciences, Hampton, VA, USA.
- 949 Centro de Biología Molecular Severo Ochoa, Consejo Superior de Investigaciones Científicas, Universidad Autónoma de Madrid, Madrid, Spain; Department of Cell Biology and Immunology, Campus de Cantoblanco, Madrid, Spain.
- 950 RIKEN, Center for Sustainable Resource Science, Wako, Saitama, Japan.
- 951 Danish Cancer Society Research Center, Center for Autophagy, Recycling and Disease, Cell Death and Metabolism Unit, Copenhagen, Denmark; University of Copenhagen, Department of Cellular and Molecular Medicine, Copenhagen, Denmark.
- 952 University of Technology, Department of applied science, Division of Biotechnology, Baghdad, Iraq.
- 953 University of Maryland School of Medicine, Department of Microbiology and Immunology, Baltimore, MD, USA.
- 954 Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Unidad de Bioquimica, Mexico City, Mexico.
- 955 University of Warwick, School of Life Sciences, Coventry, UK.
- 956 Université Paris-Saclay, INSERM ,UMR-S 1193, Châtenay-Malabry, France.
- 957 Lewis Katz School of Medicine at Temple University, Center for Translational Medicine, Philadelphia, PA, USA.
- 958 Houston Methodist Research Institute, Weill-Cornell Medicine, Houston, TX, USA.
- 959 Delft University of Technology, Kavli Institute of Nanoscience, Department of Bionanoscience, Delft, The Netherlands.
- 960 Lund University, Department of Experimental Medical Science, Laboratory of Molecular Neurogenetics, Wallenberg Neuroscience Center and Lund Stem Cell Center, Lund, Sweden.
- 961 Luxembourg Institute of Health, Tumor Immunotherapy and Microenvironment (TIME) group, Department of Oncology, Luxembourg City, Luxembourg.
- 962 Cancer Drug Resistance and Stem Cell Program, University of Sydney, NSW, Australia.
- 963 University Hospital of Regensburg, Institute of Clinical Microbiology and Hygiene, Regensburg , Germany.
- 964 Polish Academy of Sciences, Mossakowski Medical Research Centre, Laboratory of Ischemic and Neurodegenerative Brain Research, Warsaw, Poland.
- 965 Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan; Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
- 966 Université de Sherbrooke, Département d'immunologie et de biologie cellulaire, Sherbrooke, Québec, Canada.
- 967 UMR7242, CNRS, Université de Strasbourg, Strasbourg, France.
- 968 Institute of Experimental Medicine CAS, Department of Neuroregeneration, Prague, Czech Republic.
- 969 Albert Einstein College of Medicine, Department of Developmental and Molecular Biology and Department of Genetics, New York, NY, USA.
- 970 University of Copenhagen, Department of Nutrition, Exercise and Sports, Section of Molecular Physiology, Copenhagen, Denmark.
- 971 Aarhus University Hospital, Steno Diabetes Center Aarhus, Aarhus, Denmark.
- 972 University of Texas Southwestern Medical Center, Department of Molecular Biology, Dallas, TX, USA.
- 973 The First Affiliated Hospital of Nanjing Medical University, Department of Neurosurgery, Nanjing, Jiangsu, China.
- 974 Shanghai University of Traditional Chinese Medicine, Longhua Hospital, Cancer Institute, Shanghai, China.
- 975 Purdue University, Department of Nutrition Science, West Lafayette, IN, USA.
- 976 Karolinska Institutet, Department of Neurobiology, Care Sciences and Society, Division of Neurogeriatrics, Solna, Stockholm, Sweden.
- 977 The First Hospital of Jilin University, Department of Ear, Nose and Throat, Changchun, Jilin, China.
- 978 Nanjing Medical University, Nanjing First Hospital, Department of Neurology, Nanjing, China.
- 979 University of Pittsburgh, Department of Pharmacology and Chemical Biology, Pittsburgh, PA, USA.
- 980 King's College London, Institute of Psychiatry, Psychology & Neuroscience, Department of Basic and Clinical Neuroscience, Maurice Wohl Clinical Neuroscience Institute, London, UK.
- 981 Wonkwang Univesity, Department of Biological Sciences, Iksan, Chunbuk, Korea.
- 982 The First Hospital of Jilin University, Cancer Center, Department of Hematology, Changchun, Jilin, China.
- 983 ZhejiangUniversity, Sir Run Run Shaw Hospital, School of Medicine, Key Lab of Biotherapy in Zhejiang Province, Hangzhou, China.
- 984 Xi'an Jiaotong University, School of Medicine, the First Affiliated Hospital, Department of Nephrology, Xi'an City, Shaanxi Province, China.
- 985 Huazhong University of Science and Technology, Liyuan hospital of Tongji Medical College, Department of Endocrinology, Wuhan, Hubei, China.
- 986 Chungnam National University School of Medicine, Infection Control Convergence Research Center, Daejeon, Korea.
- 987 Cancer Research Center of Toulouse (CRCT), INSERM U1037, CNRS ERL5294, University of Toulouse, Toulouse, France.
- 988 University of Rochester, Department of Anesthesiology and Perioperative Medicine, Rochester, NY, USA.
- 989 University of Sheffield, Department of Infection, Immunity and Cardiovascular Disease and the Bateson Centre, Sheffield, UK.
- 990 University of Helsinki, Institute of Biotechnology, Cell and Tissue Dynamics Programme and Electron Microscopy Unit, Helsinki, Finland.
- 991 Indian Institute of Science, Centre for BioSystems Science and Engineering, Bangalore, India.
- 992 Radboud University Medical Centre, Department of Internal Medicine, Nijmegen, The Netherlands.
- 993 Universidad Castilla La Mancha, Departmento Ciencias Medicas, Albacete, Spain.
- 994 Fudan University School of Pharmacy, Department of Biological Medicines, Shanghai, China.
- 995 The University of Suwon, Department of Health Science, Hwaseong, Gyeonggi, Korea.
- 996 Stony Brook University, Department of Pathology, Stony Brook, NY, USA.
- 997 Instituto Nacional de Enfermedades Respiratorias Isamel Cosío Villegas, Departamento de Investigación en Microbiología, Ciudad de México, México.
- 998 Gwangju Institute of Science and Technology, School of Life Sciences, Gwangju, South Korea.
- 999 Korea Food Research Institute, Research Group of Natural Material and Metabolism, Jeollabuk-do, Korea.
- 1000 Ewha Womans University, Department of Biochemistry, College of Medicine, Seoul, Korea.
- 1001 Seoul National University, School of Biological Science, Seoul, Korea.
- 1002 Evelina Children's Hospital, Guy's & St Thomas' NHS Foundation Trust, Children's Neuroscience Centre, London, UK; King's College London, Muscle Signalling Section, Randall Division of Cell and Molecular Biophysics, London, UK; King's College London, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), Department of Basic and Clinical Neuroscience, London, UK.
- 1003 Memorial Sloan Kettering Cancer Center, New York, NY, USA.
- 1004 University of Ulm, Department of Internal Medicine II, Molecular Cardiology, Ulm, Germany.
- 1005 University of Eastern Finland and Kuopio University Hospital, Department of Ophthalmology, Kuopio, Finland.
- 1006 University Tartu, Department of Pharmacology, Tartu, Estonia.
- 1007 University Medical Center Gottingen, Department of Experimental Neurodegeneration, Gottingen, Germany.
- 1008 University of Michigan, Kellogg Eye Center, Department of Ophthalmology and Visual Sciences, Ann Arbor, MI, USA.
- 1009 University of California, San Francisco UCSF Diabetes Center, Department of Cell and Tissue Biology, San Francisco, CA, USA.
- 1010 University of Kansas Medical Center, Department of Microbiology, Molecular Genetics and Immunology, Kansas City, KS, USA.
- 1011 Regional Centre for Biotechnology, NCR Biotech Science Cluster, Faridabad, India.
- 1012 National Research Council of Italy (CNR), Neuroscience Institute, Padua, Italy.
- 1013 European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI), Wellcome Genome Campus, Hinxton, Cambridge, UK.
- 1014 Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland.
- 1015 Karolinska Institutet, Department of Oncology-Pathology, Stockholm, Sweden.
- 1016 Gifu University Graduate School of Medicine, Department of Cardioligy, Gifu, Japan.
- 1017 Shimane University Faculty of Medicine, Internal Medicine 1, Izumo, Shimane, Japan.
- 1018 Seoul National University, School of Biological Sciences, Seoul, Korea.
- 1019 UT Southwestern Medical Center, Department of Surgery, Dallas, Texas, USA.
- 1020 Chungbuk National University, Department of Biology Education, Seowon-Gu, Cheongju, Chungbuk, Korea.
- 1021 CAESAR Research Center, Bonn, Germany.
- 1022 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany.
- 1023 Niigata University Graduate School of Medical and Dental Sciences, Department of Cellular Physiology, Niigata, Japan.
- 1024 Department of Biomedical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA, USA.
- 1025 Florida Atlantic University, Charles E. Schmidt College of Medicine, Department of Biomedical Science, Boca Raton, FL, USA.
- 1026 Semmelweis University, Budapest, Hungary.
- 1027 National and Kapodistrian University of Athens, Medical School, Division of Molecular Oncology, Department of Biological Chemistry, Athens, Greece.
- 1028 University of Minnesota, Institute for Translational Neuroscience, Department of Neuroscience, Minneapolis, MN, USA.
- 1029 Jadavpur University, Department of Life Science and Biotechnology, Kolkata, India.
- 1030 University of Otago, Department of Physiology-HeartOtago, Dunedin, Otago, New Zealand.
- 1031 Hokkaido University, Department of Rheumatology, Endocrinology and Nephrology, Sapporo, Japan.
- 1032 Max Planck Institute for Infection Biology, Berlin, Germany.
- 1033 University of Eastern Finland, Faculty of Health Sciences, School of Pharmacy, Kuopio, Finland.
- 1034 University of Arkansas for Medical Sciences, Little Rock, AR, USA.
- 1035 Doshisha Women's College of Liberal Arts, Faculty of Pharmaceutical Sciences, Kyotanabe, Kyoto, Japan.
- 1036 Commonwealth Scientific and Industrial Research Organization (CSIRO), Agriculture and Food, Brisbane, Queensland, Australia.
- 1037 Chang Gung University, Department of Biochemistry and Molecular Biology, Taoyuan, Taiwan, Republic of China.
- 1038 Flinders University, Flinders Health and Medical Research Institute, Adelaide, Australia.
- 1039 Philipps University and University Hospital of Marburg, Department of Neuropathology, Marburg, Germany.
- 1040 National Institutes of Health, National Institute of Allergy and Infectious Diseases, Laboratory of Immunoregulation, Bethesda, MD, USA.
- 1041 Oregon Health & Science University, Casey Eye Institute, Portland, OR, USA.
- 1042 University of Münster, University Hospital, Department of Neurology with Institute of Translational Neurology, Münster, Germany.
- 1043 University of Illinois at Urbana-Champaign, Department of Molecular and Integrative Physiology, Urbana, IL, USA.
- 1044 Weill Cornell Medicine, Departments of Pediatrics and Cell and Developmental Biology, New York, NY, USA.
- 1045 Metabolomics and Cell Biology Platforms, Gustave Roussy Comprehensive Cancer Institute, Villejuif, France; Equipe 11 labellisée Ligue contre le Cancer, Centre de Recherche des Cordeliers, INSERM U 1138, Paris, France.
- 1046 Queen Mary University of London, Barts Cancer Institute, London, UK.
- 1047 University College London, MRC Laboratory for Molecular Cell Biology, London, UK.
- 1048 Maastricht University Medical Center, GROW School for Oncology, Department of Radiotherapy, Maastricht|, The Netherlands.
- 1049 Ben-Gurion University of the Negev, Faculty of Health Sciences, Department of Clinical Biochemistry and Pharmacology, Beer Sheva, Israel.
- 1050 University of Sadat City, Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, Sadat City, Egypt.
- 1051 Indiana University-Purdue University (IUPUI), Department of Pathology and Laboratory Medicine, Indianapolis, IN, USA.
- 1052 University of Eastern Finland, School of Pharmacy, Kuopio, Finland.
- 1053 Barkatullah University, Department of Biochemistry & Genetics, Bhopal, India.
- 1054 Charité - Universitätsmedizin Berlin, Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Berlin, Germany.
- 1055 Columbia University, Vagelos College of Physicians and Surgeons, Department of Pathology and Cell Biology, New York, NY, USA.
- 1056 Friedrich-Schiller-University Jena, University Hospital Jena, Institute of Human Genetics, Jena, Germany.
- 1057 Gyeongsang National University School of Medicine, Department of Biochemistry and Convergence Medical Sciences and Institude of Health Sciences, Jinju, Republic of Korea.
- 1058 University of Minnesota, Department of Biochemistry, Molecular Biology, and Biophysics, Minneapolis, MN, USA.
- 1059 Kyungpook National University, School of Medicine, Department of Biochemistry and Cell Biology, Cell and Matrix Research Institute, Daegu, Korea.
- 1060 Daegu Gyeongbuk Institute of Science and Technology (DGIST), Department of Brain and Cognitive Sciences, Daegu, Korea.
- 1061 Dankook University, College of Medicine, Department of Pharmacology, Cheonan, Chungnam, Korea.
- 1062 Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cell Factory Research Center, Daejeon, Korea; University of Science and Technology, KRIBB School of Biotechnology, Department of Environmental Biotechnology, Daejeon, Korea.
- 1063 Dankook University, College of Dentistry, Cheonan, Korea.
- 1064 Seoul National University College of Medicine, Department of Biomedical Sciences, and Ophthalmology, Seoul, Korea.
- 1065 Konkuk University, Department of Stem Cell and Regenerative Biotechnology, Seoul, Korea.
- 1066 Kyung Hee University, Department of Oral Biochemistry and Molecular Biology, School of Dentistry, Seoul, Korea.
- 1067 Sookmyung Women's University, Department of Biological Sciences, Seoul, Korea.
- 1068 Korea Research Institute of Chemical Technology, Center for Convergent Research of Emerging Virus Infection, Daejeon, Korea.
- 1069 Penn State College of Medicine, Department of Cellular and Molecular Physiology, Hershey, PA, USA.
- 1070 Weizmann Institute of Science, Department of Molecular Genetics, Rehovot, Israel.
- 1071 New York University School of Medicine, Department of Radiation Oncology, Perlmutter Cancer Center, New York, NY, USA.
- 1072 National Institute of Biomedical Innovation, Health and Nutrition (NIBIOHN), Center for Rare Disease Research, Ibaraki, Osaka, Japan.
- 1073 AstraZeneca, Bioscience, Oncology R&D, Cambridge, UK.
- 1074 University College London, Institute of Healthy Ageing, Department of Genetics, Evolution & Environment, London, UK.
- 1075 University of Utah, Huntsman Cancer Institute, Salt Lake City, UT, USA; University of Utah School of Medicine, Division of Oncology, Department of Internal Medicine, Salt Lake City, UT, USA.
- 1076 Cancer Research UK Cancer Therapeutics Unit, The Institute of Cancer Research London, Sutton, UK.
- 1077 St. Boniface Hospital Albrechtsen Research Centre, Departments of Physiology and Pathophysiology, and Pharmacology and Therapeutics, Max Rady College of Medicine; Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
- 1078 Vavilov Institute of General Genetics RAS, Department of Epigenetics, Moscow, Russia.
- 1079 S&J Kishi Research Corporation, Jupiter, FL, USA.
- 1080 Nihon Pharmaceutical University, Department of Pharmaceutical and Medical Business Sciences, Bunkyoku, Tokyo, Japan.
- 1081 St. Marianna University Graduate School of Medicine, Department of Molecular Neuroscience, Kanagawa, Japan.
- 1082 Nagasaki University, Institute of Biomedical Sciences, Department of Clinical Research Pharmacy, Nagasaki, Japan.
- 1083 Albert Einstein College of Medicine, Departments of Medicine and Cell Biology and Wilf Family Cardiovascular Research Institute, Bronx, NY, USA.
- 1084 University of Copenhagen, Department of Neuroscience, Copenhagen, Denmark.
- 1085 Perelman School of Medicine at the University of Pennsylvania, Department of Medicine (Hematology-Oncology), Philadelphia, PA, USA.
- 1086 Friedrich-Baur-Institute, Department of Neurology, University of Munich, Munich, Germany.
- 1087 University Hospital Erlangen, Department of Molecular Neurology, Erlangen, Germany.
- 1088 University of Oslo, Centre for Cancer Cell Reprogramming, Institute of Clinical Medicine, Faculty of Medicine, Montebello, Norway; Oslo University Hospital, Department of Molecular Cell Biology, Institute for Cancer Research, Montebello, Norway.
- 1089 The University of Tokyo, Department of Biochemistry and Molecular Biology, Graduate School of Medicine, Tokyo, Japan.
- 1090 The Hong Kong Polytechnic University, Department of Applied Biology and Chemical Technology, Hong Kong, China.
- 1091 Icahn School of Medicine at Mount Sinai, Department of Geriatrics and Palliative Medicine, New York, NY, USA.
- 1092 The University of Tokyo, Institute for Quantitative Biosciences, Laboratory of RNA Function, Tokyo, Japan; Albert Einstein College of Medicine, Department of Anatomy and Structural Biology, Bronx, NY, USA.
- 1093 New York Institute of Technology, College of Osteopathic Medicine, Department of Biomedical Sciences, Old Westbury, NY, USA.
- 1094 Johann Wolfgang Goethe-University Frankfurt am Main, Faculty of Computer Science and Mathematics, Institute of Computer Science, Frankfurt am Main, Germany.
- 1095 University Medicine Göttingen, Clinic for Neurology, Göttingen, Germany.
- 1096 University Hospital Münster (UKM), Institute of Musculoskeletal Medicine, Münster, Germany.
- 1097 Goethe University Hospital Frankfurt, Neuroscience Center, Experimental Neurosurgery, Frankfurt/Main, Germany.
- 1098 Division of Brain Disease Research, Korea National Institute of Health, Cheongju-si, Republic of Korea.
- 1099 Juntendo University Graduate School of Medicine, Department of Cell Biology and Neuroscience, Hongo, Bunkyo-ku, Tokyo, Japan.
- 1100 University of Graz, Institute of Molecular Biosciences, BioTechMed-Graz, Graz, Austria.
- 1101 Juntendo University School of Medicine, Department of Physiology, Bunkyo-ku, Tokyo, Japan.
- 1102 Sapporo Higashi Tokushukai Hospital, Advanced Surgery Center, Sapporo, Hokkaido, Japan.
- 1103 The Abigail Wexner Research Institute at Nationwide Children's Hospital, Center for Microbial Pathogenesis, Columbus, OH, USA.
- 1104 Earlham Institute, Norwich, UK.
- 1105 Newcastle University, Ageing Research Laboratories, Institute for Cell and Molecular Biosciences, Newcastle upon Tyne, UK.
- 1106 Norwegian University of Science and Technology (NTNU), Department of Biotechnology and Food Science, Trondheim, Norway; Korsnes Biocomputing (KoBio), Trondheim, Norway.
- 1107 University of Eastern Finland, Department of Ophthalmology, Kuopio, Finland.
- 1108 Indiana University School of Medicine, Indianapolis, IN, USA.
- 1109 Hiroshima University, Graduate School of Biomedical and Health Sciences, Hiroshima, Japan.
- 1110 China National Rice Research Institute, State Key Laboratory of Rice Biology, Hangzhou. China.
- 1111 Democritus University of Thrace, Department of Radiotherapy/Oncology, Alexandroupolis, Greece.
- 1112 Kanazawa Medical University, Department of Diabetology & Endocrinology, Uchinadacho, Ishikawa, Japan.
- 1113 Fukuoka University, Faculty of Pharmaceutical Sciences, Department of Biochemistry, Fukuoka, Japan.
- 1114 University of Freiburg, Institute of Biochemistry and Molecular Biology, ZBMZ, Faculty of Medicine, Freiburg, Germany; University of Freiburg, CIBSS - Centre for Integrative Biological Signalling Studies, Freiburg, Germany.
- 1115 Northwestern University Feinberg School of Medicine, Department of Neurology, Chicago, IL, USA.
- 1116 University of Texas, Southwestern Medical Center, Dallas, TX, USA.
- 1117 Queen's University of Belfast, Wellcome-Wolfson Institute for Experimental Medicine, Belfast, UK.
- 1118 Université Lyon, Université Claude Bernard Lyon 1, CNRS UMR 5310, INSERM U1217, Institut NeuroMyoGène, Lyon, France.
- 1119 Université de Paris, Sorbonne Université, INSERM U1138, Centre de Recherche des Cordeliers, Equipe labellisée par la Ligue contre le cancer, Paris, France; Institut Gustave Roussy, Metabolomics and Cell Biology Platforms, Villejuif, France; Hôpital Européen Georges Pompidou, AP-HP, Pôle de Biologie, Paris, France; Chinese Academy of Medical Sciences, Suzhou Institute for Systems Medicine, Suzhou, China; Karolinska Institute, Karolinska University Hospital, Department of Women's and Children's Health, Stockholm, Sweden.
- 1120 Babraham Institute, Babraham, Cambridge, UK.
- 1121 Tokyo University of Science, Department of Applied Biological Science, Noda, Chiba, Japan.
- 1122 VIB-KU Leuven Center for Brain & Disease Research, Leuven, Belgium; KU Leuven, Department of Neurosciences, Leuven Brain Institute, Leuven, Belgium.
- 1123 University of Bonn, LIMES Institute, Bonn, Germany.
- 1124 Emory University, Department of Pharmacology and Chemical Biology, School of Medicine, Atlanta, GA, USA.
- 1125 PK-PD-Toxicology and Formulation Division, CSIR-Indian Institute of Integrative Medicine, Jammu, India.
- 1126 University of Houston, Department of Pharmacological and Pharmaceutical Sciences, Houston, TX, USA.
- 1127 Julius L. Chambers Biomedical/Biotechnology Research Institute (BBRI) and North Carolina Central University, Department of Pharmaceutical Sciences, Durham, NC, USA.
- 1128 International Centre for Genetic Engineering and Biotechnology, Cellular Immunology Group, Aruna Asaf Ali Marg, New Delhi, India.
- 1129 Shiga University of Medical Science, Department of Medicine, Otsu, Shiga, Japan.
- 1130 Kalinga institute of industrial technology (KIIT-DU), School of Biotechnology, Campus 11, Patia, Bhubaneswar, Odisha, India.
- 1131 St. Jude Children's Research Hospital, Department of Pathology, Memphis, TN, USA.
- 1132 Indian Institute of Technology (IIT) Guwahati, Department of Biosciences and Bioengineering, Guwahati, Assam, India.
- 1133 Virginia Commonwealth University, Department of Computer Science, Richmond, VA, USA.
- 1134 University of Colorado Anschutz Medical Campus, Department of Pharmacology, Aurora, CO, USA.
- 1135 Sabanci University Nanotechnology Research and Application Center (SUNUM), Tuzla, Istanbul, Turkey.
- 1136 Wonkwang University School of Medicine, Zoonosis Research Center, Iksan, Jeonbuk, Korea.
- 1137 Keimyung University, School of Medicine, Department of Immunology, Daegu, Korea.
- 1138 Protein Metabolism Medical Research Center and Department of Biomedical Sciences, Seoul National University School of Medicine, Seoul, Korea.
- 1139 University of California, Irvine School of Medicine, Irvine, CA, USA.
- 1140 INRS, INRS-Institut Armand-Frappier. Montréal, QC, Canada.
- 1141 University of Burgundy, Centre Georges François Leclerc, Department of Medical Oncology, Dijon, France.
- 1142 University of Ferrara, Department of Translational Medicine, Ferrara, Italy.
- 1143 Cellular Microbiology and Physics of Infection Group, Center for Infection and Immunity of Lille, Institut Pasteur de Lille, CNRS, INSERM, CHU Lille, University of Lille, Lille Cedex, France.
- 1144 University of Ottawa, Department of Cellular & Molecular Medicine, Ottawa, Ontario, Canada.
- 1145 Huazhong Agricultural University, National Key Laboratory of Crop Genetic Improvement, Wuhan, China.
- 1146 Macquarie University, Faculty of Medicine, Health and Human Sciences, Department of Biomedical Science, Centre for Motor Neuron Disease Research, Sydney, NSW, Australia.
- 1147 University of California, San Francisco Department of Ophthalmology, San Francisco, CA, USA.
- 1148 National Yang-Ming University, Department of Life Sciences and Institute of Genome Sciences, Taipei, Taiwan.
- 1149 Yale University, Department of Cellular and Molecular Physiology, New Haven, CT, USA.
- 1150 School of Biochemistry, University of Bristol, Bristol, UK.
- 1151 Penn State College Medicine, Department of Cellular and Molecular Physiology, Hershey, PA, USA.
- 1152 University of Greifswald, Institute of Pharmacy, Greifswald, Germany.
- 1153 Stockholm University, Department of Biochemistry and Biophysics, Stockholm, Sweden.
- 1154 Johannes Kepler University Linz, Kepler University Hospital GmbH, Institute of Pathology and Molecular Pathology, Linz, Austria.
- 1155 University Bourgogne Franche-Comté, AgroSup Dijon, PAM UMR A 02.102, Dijon, France.
- 1156 IGBMC, Inserm U1258, Cnrs UMR7104, Strasbourg University, Illkirch, France.
- 1157 Mayo Clinic, Department of Medicine, Division of Gastroenterology and Hepatology, Rochester, MN, USA.
- 1158 University of Virginia, Department of Cell Biology, Charlottesville, VA, USA.
- 1159 Macau University of Science and Technology, State Key Laboratory of Quality Research in Chinese Medicine, Taipa, Macau, China.
- 1160 The Hong Kong Polytechnic University, Faculty of Health and Social Sciences, Department of Health Technology and Informatics, Hunghom, Hong Kong.
- 1161 University of Nottingham, School of Life Sciences, Nottingham, UK.
- 1162 Sichuan Academy of Medical Science & Provincial Hospital, Medical School of UESTC, Chengdu, China.
- 1163 Université Paris Saclay, Institut des Neurosciences Paris-Saclay (Neuro-PSI)-CNRS UMR 9197, Orsay France.
- 1164 McGill University Health Center, Department of Medicine, Cancer Research Program, Montreal, QC, Canada.
- 1165 Research Institute of the McGill University Health Centre, Meakins-Christie Laboratories and Translational Research in Respiratory Diseases Program, Montréal, Québec, Canada; McGill University, Department of Critical Care and Division of Experimental Medicine, Montréal, Québec, Canada; UQAM, Faculté des Sciences, Département des Sciences de l'activité physique, Montréal, Canada.
- 1166 Ulsan National Institute of Science and Technology, Department of Biological Sciences, Ulsan, Korea.
- 1167 National Taipei University of Nursing and Health Sciences, School of Nursing, Taipei, Taiwan.
- 1168 KAIST, Department of Biological Sciences, Daejon, Korea.
- 1169 Konkuk University, School of Medicine, Department of Anatomy, Seoul, Korea.
- 1170 Korea Advanced Institute of Science and Technology, Graduate School of Medical Science and Engineering, Daejeon, Korea.
- 1171 QIMR Berghofer Medical Research Institute, Herston, Queensland, Australia.
- 1172 Hannam University, Department of Biological Sciences and Biotechnology, Daejeon, South Korea.
- 1173 Chungnam National University, Graduate School of Analytical Science and Technology (GRAST), Daejeon 305-764, Republic of Korea.
- 1174 University of Michigan, Department of Molecular & Integrative Physiology, Ann Arbor, MI, USA.
- 1175 Incheon National University, College of Life Sciences and Bioengineering, Division of Life Sciences, Incheon, Korea.
- 1176 Yonsei University College of Medicine, Department of Pharmacology, Seoul, Korea.
- 1177 Seoul National University College of Medicine, Department of Biochemistry and Molecular Biology, Seoul, Korea.
- 1178 Yonsei University College of Medicine, Severance Biomedical Science Institute and Department of Internal Medicine, Seoul, Korea.
- 1179 Ajou University Graduate School of Medicine, Department of Biomedical Sciences, Suwon, Gyeonggi, Korea.
- 1180 Seoul National University College of Medicine, Department of Biomedical Sciences, Neuroscience Research Institute, Seoul, Korea.
- 1181 University of Kansas Medical Center, Department of Biochemistry and Molecular Biology, Kansas City, KS, USA.
- 1182 DGIST, Department of Brain & Cognitive Sciences, Daegu, Korea.
- 1183 University of Arizona College of Medicine, Department of Basic Medical Sciences, Phoenix, AZ, USA.
- 1184 Ditmanson Medical Foundation Chia-Yi Christian Hospital, Translational Medicine Research Center, Chiayi City, Taiwan.
- 1185 Yonsei University College of Medicine, Department of Internal Medicine, Seoul, Korea.
- 1186 University of West Florida, Department of Movement Sciences and Health, Pensacola, FL, USA.
- 1187 University of Michigan, Rogel Cancer Center, Department of Periodontics and Oral Medicine, Department of Otolaryngology - Head and Neck Surgery, Ann Arbor, MI, USA.
- 1188 National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
- 1189 University of Maribor, Faculty of Medicine, Maribor, Slovenia; University of Maribor, Faculty of Natural Sciences and Mathematics, Department of Biology, Maribor, Slovenia; University of Maribor, Faculty of Chemistry and Chemical Engineering, Maribor, Slovenia; Medical University of Graz, Division of Cell Biology, Histology and Embryology, Gottfried Schatz Research Center, Graz, Austria.
- 1190 Medical University of Graz, Division of Cell Biology, Histology and Embryology, Gottfried Schatz Research Center, Graz, Austria.
- 1191 Guangzhou Municipal and Guangdong Provincial Key Laboratory of Protein Modification and Degradation, School of Basic Medical Sciences, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, China.
- 1192 Institut National de la Santé et de la Recherche Médicale (Inserm), Université de Paris, Paris Cardiovascular Center - PARCC, Paris, France.
- 1193 Universidade Federal do Rio Grande do Sul (UFRGS), Center of Biotechnology and Department of Biophysics, Porto Alegre, RS, Brazil.
- 1194 USC Norris Comprehensive Cancer Center , Los Angeles, CA, USA.
- 1195 University of Pisa, Department of Translational Research and New Technologies in Medicine and Surgery, Pisa, Italy.
- 1196 Universidad Autónoma de Madrid, Department of Biology, Madrid, Spain.
- 1197 University of São Paulo, School of Pharmaceutical Sciences of Ribeirão Preto, Department of Clinical Analyses, Toxicology and Food Sciences, Ribeirão Preto, SP, Brazil.
- 1198 University of Luxembourg, Department of Life Sciences and Medicine, Molecular Disease Mechanisms Group, Belval, Luxembourg.
- 1199 The Chinese University of Hong Kong, Faculty of Medicine, School of Biomedical Sciences, Shatin, New Territories, Hong Kong.
- 1200 Icahn School of Medicine at Mount Sinai, Department of Medicine, New York, NY, USA.
- 1201 Royal Veterinary College, London, UK; UCL Queen Square Institute of Neurology, Department of Neurodegenerative Disease, London, UK.
- 1202 La Jolla Institute for Immunology and University of California San Diego, Laboratory of Inflammation Biology, La Jolla, CA, USA.
- 1203 College of Life Science and Technology, Jinan University, Guangzhou, China.
- 1204 Fudan University, Shanghai Cancer Center and Institutes of Biomedical Sciences, Shanghai, China.
- 1205 Sun Yat-sen University, Sun Yat-sen Memorial Hospital, Department of Pathology, Guangzhou, Guangdong Province, China.
- 1206 Sichuan University, West China Hospital, State key laboratory of biotherapy, Chengdu, Sichuan, China.
- 1207 Virginia Polytechnic Institute, Department of Biological Sciences, Blacksburg, VA, USA.
- 1208 Children's Hospital of Soochow University, Institute of Pediatric Research, Suzhou, China.
- 1209 Department of Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, MI, USA.
- 1210 Jinan University, College of Life Science and Technology, Department of Biology, Guangzhou, China.
- 1211 Virginia Commonwealth University, Departement of Pharmacology and Toxicology, Richmond, VA, USA.
- 1212 Fudan University, Hospital of Obstetrics and Gynecology, Institute of Obstetrics and Gynecology, Laboratory for Reproductive Immunology, Shanghai, China.
- 1213 South China Normal University, School of Life Sciences, Institute of Insect Science and Technology, Guangdong Provincial Key Laboratory of Insect Developmental Biology and Applied Technology, Guangzhou, China.
- 1214 Emory University School of Medicine, Department of Pharmacology and Chemical Biology, Atlanta, GA, USA.
- 1215 Boston Children's Hospital, Harvard Medical School, Departments of Urology and Surgery, Boston, MA, USA.
- 1216 Stanford University, Department of Gastroenterology & Hepatology, Stanford, CA, USA.
- 1217 Chinese Academy of Agricultural Sciences, Institute of Plant Protection, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Beijing, China.
- 1218 Chinese Academy of Agriculture Sciences, Lanzhou Veterinary Research Institute, State Key Laboratory of Veterinary Etiological Biology, Lanzhou, Gansu, China.
- 1219 Chinese Academy of Sciences, Hefei Institutes of Physical Science, High Magnetic Field Laboratory, Hefei, Anhui Province, China.
- 1220 Army Medical University, Department of Bichemistry and Molecular Biology, Chongqing, Chongqing, China.
- 1221 The Wistar Institute, Molecular and Cellular Oncogenesis Program, Philadelphia, PA, USA.
- 1222 Sun Yat-sen University, The 3rd Affiliated Hospital of Sun Yat-sen University, Vaccine Research Institute, Guangzhou, China.
- 1223 Nanjing Agricultural University, College of Life Sciences, Key Laboratory of Agricultural Environmental Microbiology of Ministry of Agriculture and Rural Affairs, Nanjing , China.
- 1224 Free University of Berlin, Department of Biology, Chemistry and Pharmacy, Berlin, Germany.
- 1225 Northwest A&F University, College of Life Sciences, Yangling, Shaanxi, China.
- 1226 Academia Sinica, Institute of Cellular and Organismic Biology, Taipei, Taiwan.
- 1227 Nathan S. Kline Institute, Center for Dementia Research, Orangeburg, NY, USA.
- 1228 National Institute of Child Health and Human Development, National Institutes of Health, Cell Biology and Neurobiology Branch, Bethesda, MD, USA.
- 1229 Konkuk University, Department of Veterinary Medicine, Seoul, Korea.
- 1230 São Paulo State University, Botucatu Medical School, Center for Evaluation of Environmental Impact on Human Health (TOXICAM), Department of Pathology, Botucatu, SP, Brazil.
- 1231 San Raffaele Open University and IRCCS San Raffaele Pisana, Laboratorio di Patologia Cellulare e Molecolare, Rome, Italy.
- 1232 First Affiliated Hospital of Nanjing Medical University, Department of Neurosurgery, Nanjing, China; Jiangsu Province Hospital, Department of Neurosurgery, Nanjing, China.
- 1233 Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-Da Hospital, Kaohsiung, Taiwan; I-Shou University, College of Medicine, School of Medicine, Kaohsiung, Taiwan.
- 1234 Mackay Memorial Hospital, Department of Pediatrics, Taipei, Taiwan; Mackay Medical College, Department of Medicine, New Taipei,Taiwan.
- 1235 Zhejiang Academy of Agricultural Sciences, Institute of Plant Protection and Microbiology, State Key Laboratory for Managing Biotic and Chemical Treatments to the Quality and Safety of Agro-products, Hangzhou, China; Zhejiang University, Institute of Biotechnology, Hangzhou, China.
- 1236 Life Sciences Institute and Department of Cell & Developmental Biology, University of Michigan, Ann Arbor, MI, USA.
- 1237 National Health Research Institutes, Institute of Biomedical Engineering and Nanomedicine, Zhunan, Miaoli, Taiwan.
- 1238 Chang Gung Memorial Hospital, Liver Research Center, Linkou, Taoyuan, Taiwan; Chang Gung University, College of Medicine, Graduate Institute of Biomedical Sciences, Taoyuan, Taiwan; Chang Gung University of Science and Technology, College of Human Ecology, Research Center for Chinese Herbal Medicine, Taoyuan, Taiwan.
- 1239 The Ohio State University, Wexner Medical Center, Department of Surgery, Columbus, OH, USA.
- 1240 School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, China.
- 1241 Huazhong University of Science and Technology, College of Life Science and Technology, Hubei Bioinformatics and Molecular Imaging Key Laboratory, Key Laboratory of Molecular Biophysics of Ministry of Education, Wuhan, Hubei, China.
- 1242 Harvard Medical School, Massachusetts General Hospital, Gastrointestinal Unit/Medicine, Boston, MA, USA.
- 1243 Mayo Clinic, Department of Biochemistry and Molecular Biology, Rochester, MN, USA.
- 1244 Brigham and Women's Hospital, Harvard Medical School, Center for Nanomedicine and Department of Anesthesiology, Boston, MA, USA.
- 1245 Instituto de Biofisica da UFRJ, Rio de Janeiro, Brazil.
- 1246 Technical University of Munich, School of Medicine, Klinikum rechts der Isar, Department of Neurology, Munich, Germany.
- 1247 Indiana University School of Medicine, Department of Pediatrics and Center for Diabetes and Metabolic Diseases, Indianapolis, IN, USA.
- 1248 University of Singapore, Department of Biological Sciences, Singapore.
- 1249 University of Maryland School of Medicine, Department of Anesthesiology, Baltimore, MD, USA.
- 1250 University of Iowa, Fraternal Order of Eagles Diabetes Research Center, Obesity Research and Education Initiative, Abboud Cardiovascular Research Center, Department of Health and Human Physiology, Iowa City, IA, USA.
- 1251 Poznan University of Medical Sciences, Department of Clinical Chemistry and Molecular Diagnostics, Poznan, Poland.
- 1252 Duke University, Department of Ophthalmology, Durham, NC, USA.
- 1253 Department of Microbiology & Immunology, Dalhousie University, Halifax, Nova Scotia, Canada.
- 1254 Institute of Neuroscience of Soochow University, Suzhou, China.
- 1255 Chinese Academy of Sciences, Center for Biosafety Mega-Science, Institute of Microbiology, CAS Key Laboratory of Pathogenic Microbiology and Immunology, Chaoyang District, Beijing, China.
- 1256 Nankai University, Department of Microbiology, Tianjin, China.
- 1257 Guangdong Medical University, Institute of Nephrology, and Zhanjiang Key Laboratory of Prevention and Management of Chronic Kidney Disease, Zhanjiang, Guangdong, China.
- 1258 Capital Medical University, Beijing Institute for Brain Disorders, Beijing, China.
- 1259 Central South University, School of Life Sciences, Molecular Biology Research Center, Changsha, Hunan, China.
- 1260 The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- 1261 Columbia University, Naomi Berrie Diabetes Center, Department of Pathology and Cell Biology, College of Physicians and Surgeons, New York, NY, USA.
- 1262 University of New Mexico Health Sciences Center, Department of Biochemistry and Molecular Biology, Albuquerque, NM, USA.
- 1263 Henan Academy of Agricultural Sciences, Institute of Plant Nutrition, Agricultural Resources and Environmental Science, Jinshui District, Zhengzhou, Henan, China.
- 1264 Zhejiang University School of Medicine, Department of Biochemistry and Department of Cardiology of the Second Affiliated Hospital, Hangzhou, Zhejiang, China.
- 1265 Zhejiang University, Biotechnology Institute, State Key Laboratory for Rice Biology, Hangzhou, China.
- 1266 CAS Key Laboratory of Regenerative Biology, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China.
- 1267 Harvard Medical School, Department of Microbiology; Brigham and Women's Hospital, Division of Infectious Diseases, Boston, MA, USA.
- 1268 University of Colorado at Boulder, Department of Biochemistry, Boulder, CO, USA.
- 1269 ShanghaiTech University, School of Life Science and Technology, Shanghai, China.
- 1270 Saarland University, Department of Neurology, Homburg, Germany.
- 1271 Johns Hopkins University, Department of Biochemistry and Molecular Biology, Baltimore, MD, USA.
- 1272 Shenyang Pharmaceutical University, Department of Pharmacology, Shenyang, China.
- 1273 Tsinghua Unversity, School of Life Sciences, Beijing, China.
- 1274 University of Texas MD Anderson Cancer Center, Department of Sarcoma Medical Oncology, Houston, TX, USA.
- 1275 University of Bourgogne Franche-Comté, Team 'Biochemistry of the Peroxisome, Inflammation and Lipid Metabolism (EA7270)/Inserm, Faculty of Sciences Gabriel, Dijon, France.
- 1276 Universitat Autonoma de Barcelona, Departament of Biochemistry and Institut Neurosciences, Faculty of Medicine, Bellaterra, Barcelona, Spain.
- 1277 Vall d´Hebron Research Institute, Barcelona, Spain.
- 1278 Oslo University Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Oslo, Norway.
- 1279 Harvard Medical School, Brigham and Women's Hospital, Department of Neurology, Boston, MA, USA.
- 1280 London School of Hygiene & Tropical Medicine, Department of Infection Biology, London, UK.
- 1281 Universidad Autonoma de Madrid, School of Medicine, Department of Pharmacology, Madrid, Spain.
- 1282 Universidad Autónoma de Madrid, Departamento de Biología Molecular, Madrid, Spain and Centro de Biología Molecular Severo Ochoa (CSIC-UAM), Madrid, Spain.
- 1283 Universidad de Zaragoza, IA2, IIS, Laboratorio de Genética Bioquímica (LAGENBIO), Zaragoza, Spain; Universidad de Zaragoza, IA2, IIS, Centro de Encefalopatías y Enfermedades Transmisibles Emergentes, Zaragoza, Spain.
- 1284 University Medical Center Utrecht, Center for Molecular Medicine, Utrecht, The Netherlands; Regenerative Medicine Center, Utrecht, The Netherlands.
- 1285 University of Turin, Department of Veterinary Sciences, Grugliasco (TO), Italy.
- 1286 Université de Paris, Centre de Recherche sur l'Inflammation (CRI), Inserm-UMR1149, Paris, France.
- 1287 Newcastle University, Institute of Translation and Clinical Studies, Precision Medicine, The Medical School, Newcastle upon Tyne, UK.
- 1288 State University of New York at Buffalo, Buffalo, NY, USA.
- 1289 Universidad Mayor, Center for Integrative Biology and Geroscience for Brain, Health and Metabolism, Santiago, Chile.
- 1290 National University of Singapore, Yong Loo Lin School of Medicine, Department of Physiology, Singapore.
- 1291 Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA.
- 1292 University of Macau, Institute of Chinese Medical Sciences, State Key Laboratory of Quality Research in Chinese Medicine, Taipa, Macao, China.
- 1293 Institute of Virology, University Hospital Essen, Essen, Germany.
- 1294 Pfizer Inc, DSRD, La Jolla, CA, USA.
- 1295 University of St Andrews, School of Medicine, North Haugh, St Andrews, UK.
- 1296 Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal and ICVS/3B's - PT Government Associate Laboratory, Braga/Guimarães, Portugal.
- 1297 Duke University, School of Medicine, Duke Center for Virology, Department of Molecular Genetics and Microbiology, Durham, NC, USA.
- 1298 BC Cancer, Trev and Joyce Deeley Research Centre, Victoria, BC, Canada; University of Victoria, Department of Biochemistry and Microbiology, Victoria, BC, Canada.
- 1299 University of Würzburg, Institute of Clinical Neurobiology, Würzburg, Germany.
- 1300 University of British Columbia, Centre for Heart Lung Innovation/Department of Pathology and Laboratory Medicine, Vancouver, BC, Canada.
- 1301 Kunming Institute of Zoology, Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences & Yunnan Province, Kunming, Yunnan, China.
- 1302 Plymouth University, Peninsula School of Medicine and Dentistry, Plymouth, UK.
- 1303 Huazhong Agricultural University, Laboratory of Molecular Nutrition for Aquatic Economic Animals, Fishery College, Wuhan, China.
- 1304 RWTH Aachen University, Medical School, Institute of Biochemistry and Molecular Biology, Aachen, Germany.
- 1305 International Clinical Research Center, St'Anne University Hospital, Brno, Czech Republic; Institute of Molecular Biology and Biophysics, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Siberia, Russia.
- 1306 University of Oslo, Faculty of Medicine, Institute of Basic Medical Sciences, Department of Molecular Medicine, Oslo, Norway; University of Oslo, Faculty of Medicine, Institute of Clinical Medicine, Centre for Cancer Cell Reprogramming, Oslo, Norway.
- 1307 Justus Liebig University, University Hospital Giessen and Marburg GmbH, Departement of Ophthalmology, Giessen, Germany.
- 1308 The Hong Kong Polytechnic University, Department of Health Technology and Informatics, Hong Kong, China.
- 1309 Shandong Normal University, Shandong Provincial Key Laboratory of Plant Stress, College of Life Sciences, Jinan, Shandong, China.
- 1310 Yale School of Medicine, Department of Cell Biology, New Haven, CT, USA.
- 1311 Guangzhou Medical University, Department of Histology and Embryology, Guangzhou, China.
- 1312 Soochow University, Institute of Neuroscience, Jiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases, Suzhou, Jiangsu, China.
- 1313 Department of Emergency Medicine, Thomas Jefferson University, Philadelphia, PA, USA.
- 1314 Air Force Medical University, Xijing Hospital, Department of Clinical Laboratory, Xi'an, China.
- 1315 Tianjin Medical University, Department of Biochemistry and Molecular Biology; School of Basic Medical Sciences, Tianjin Key Laboratory of Medical Epigenetics, Tianjin, China.
- 1316 Albert Einstein College of Medicine, Department of Pathology. Bronx, NY, USA.
- 1317 Iowa State University, Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology, Ames, IA, USA.
- 1318 Michigan State University, College of Human Medicine, Grand Rapids, MI, USA.
- 1319 Ben May Department for Cancer Research, University of Chicago, Chicago IL, USA.
- 1320 Perelman School of Medicine at the University of Pennsylvania, Department of Neuroscience, Philadelphia, PA, USA.
- 1321 University of Graz, Institute of Molecular Biosciences, BioTechMed-Graz, BioHealth, Graz, Austria.
- 1322 University of Texas Health San Antonio, Department of Medicine, San Antonio, TX, USA.
- 1323 Albert Einstein College of Medicine, Institute for Aging Studies, Department of Developmental and Molecular Biology, Bronx, NY, USA.
- 1324 RIKEN, Laboratory for Retinal Regeneration, Kobe, Hyogo, Japan.
- 1325 Tokyo Medical and Dental University, Department of Cardiovascular Medicine, Tokyo, Japan.
- 1326 Justus-Liebig University, Department of Internal Medicine, Universities of Giessen & Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany.
- 1327 Part overlaps with Costanza Montagna: Danish Cancer Society Research Center, Computational Biology Laboratory, Copenhagen, Denmark; Danish Cancer Society Research Center, Computational Biology Laboratory, Copenhagen, Denmark.
- 1328 Cellular and Molecular Signaling, New York, NY, USA.
- 1329 Central Michigan University, Department of Psychology and Neuroscience Program, Mt. Pleasant, MI, USA.
- 1330 Inserm U1138, Centre de Recherche des Cordeliers, Sorbonne Université, Université de Paris, Paris, France.
- 1331 University of Naples Federico II, Department of Biology, Naples, Italy.
- 1332 Washington University in St. Louis, Department of Cell Biology and Physiology, St. Louis, MO, USA.
- 1333 CSIR - Indian Institute of Integrative Medicine, Department of Cancer Pharmacology, Sanat Nagar, Srinagar, Jammu and Kashmir, India.
- 1334 National University of Singapore, Yong Loo Lin School of Medicine, Department of Physiology, Mitochondrial Physiology and Metabolism Lab, Singapore; National University of Singapore, NUHS Centre for Healthy Ageing, Singapore.
- 1335 Center for Global Health, Catholic University of the Sacred Heart, Rome, Italy.
- 1336 Oregon Health and Science University, Knight Cardiovascular Institute, Portland, OR, USA.
- 1337 Iowa State University, Department of Biomedical Sciences, Ames, IA, USA.
- 1338 The Chinese University of Hong Kong, The Prince of Wales Hospital, The Faculty of Medicine, Department of Orthopaedics and Traumatology, New Territories, Shatin, Hong Kong, China.
- 1339 Dana-Farber Cancer Institute, Division of Genomic Stability and DNA Repair, Department of Radiation Oncology, Boston, MA, USA.
- 1340 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany; CAESAR Research Center, Bonn, Germany.
- 1341 Vita-Salute San Raffaele University & IRCCS San Raffaele Scientific Institute, Division of Immunology, Transplantation and Infectious Diseases, Milano, Italy.
- 1342 Virginia Commonwealth University School of Medicine, VCU Institute of Molecular Medicine, Massey Cancer Center, Department of Microbiology and Immunology, Richmond, VA, USA.
- 1343 Molecular Biology and Genetics Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India.
- 1344 KU Leuven, Department of Imaging and Pathology, Leuven, Belgium.
- 1345 University College London, School of Pharmacy, Department of Pharmacology, London, United Kingdom.
- 1346 Massachusetts General Hospital, Department of Molecular Biology, Boston, MA, USA.
- 1347 Université Côte d'Azur, INSERM, C3M, Nice, France.
- 1348 Presidio San Paolo Polo Universitario, Dipartimento di Scienze della Salute San Paolo, Milano, Italy.
- 1349 University of Milan, Department of Pharmacological and Biomolecular Sciences, Milan, Italy.
- 1350 Consiglio Nazionale delle Ricerche, Istituto di Biochimica e Biologia Cellulare, Monterotondo scalo (RM), Italy.
- 1351 University of Camerino, School of Pharmacy, Camerino, MC, Italy.
- 1352 University of Oviedo, Principality of Asturias Institute for Biomedical Research, Department of Functional Biology, Oviedo, Spain.
- 1353 Washington University in St. Louis, Department of Biology, St. Louis, MO, USA.
- 1354 UMBC, Department of Chemical, Biochemical and Environmental Engineering, Baltimore, MD, USA.
- 1355 University of Vienna, Max Perutz Labs, Department of Biochemistry and Cell Biology, Vienna BioCenter, Vienna, Austria.
- 1356 Harvard Medical School, Department of Biological Chemistry and Molecular Pharmacology, Boston, MA, USA.
- 1357 European Institute of Oncology (IEO) IRCCS, Department of Experimental Oncology, Milan, Italy; University of Milano, Department of Oncology and Hemato-Oncology, Milan, Italy.
- 1358 KU Leuven, Department of cellular and Molecular Medicine, Laboratory of cellular transport systems, Leuven, Belgium.
- 1359 Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), Department of Biotechnology, Madrid, Spain.
- 1360 Laboratorio de Genética Bioquímica (LAGENBIO), Centro de Encefalopatías y Enfermedades Transmisibles Emergentes, Instituto Agroalimentario de Aragón-IA2, Instituto de Investigación Sanitaria Aragón-IISA, Universidad de Zaragoza, Zaragoza, Spain; Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Instituto Carlos III, Zaragoza, Spain.
- 1361 University of Las Palmas de Gran Canaria, Department of Physical Education and Research Institute of Biomedical and Health Sciences (IUIBS), Las Palmas de Gran Canaria, Spain.
- 1362 Instituto de Investigaciones Biomédicas "Alberto Sols", CSIC-UAM, Madrid, Spain.
- 1363 Cell and Developmental Biology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
- 1364 University of Cambridge, MRC Mitochondrial Biology Unit, Cambridge, UK.
- 1365 National Institutes of Health, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA.
- 1366 Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, AC, Unidad de Biotecnología Médica y Farmacéutica, Guadalajara, Jalisco, México.
- 1367 Albert Einstein College of Medicine, Radiation Oncology, Bronx, NY, USA.
- 1368 Vall d'Hebron research Institute-UAB/Neurodegenerative Diseases Group, Barcelona, Spain.
- 1369 University of São Paulo, Institute of Biomedical Sciences, Department of Anatomy, Laboratory of Functional Neuroanatomy of Pain, São Paulo, Brazil.
- 1370 Laboratory of Immunoendocrinology, Department of Clinical and Toxicological Analyses, University of São Paulo, São Paulo, Brazil.
- 1371 Universidade Anhanguera de São Paulo, Programa de Pós-graduação Stricto sensu e Pesquisa, São Paulo, Brazil.
- 1372 Università Cattolica del Sacro Cuore, Rome, Italy.
- 1373 Institut Jean-Pierre Bourgin, INRAE, AgroParisTech, Université Paris-Saclay, 78000 Versailles, France.
- 1374 University of Minnesota, Department of Biochemistry, Molecular Biology and Biophysics and Department of Medicine, Minneapolis, MN, USA.
- 1375 Università degli Studi di Milano, Dipartimento di Scienze della Salute, Milano, Italy.
- 1376 Universidad Nacional Autónoma de México, Instituto de Fisiología Celular, División de Neurociencias, Departamento de Neuropatología Molecular, Mexico, DF, Mexico.
- 1377 MRC Laboratory of Molecular Biology, Cambridge, UK.
- 1378 University of Rome "Sapienza", Department of Experimental Medicine, Rome, Italy.
- 1379 Center for gender-specific medicine, Italian National Health Institute, Rome, Italy.
- 1380 Biodonostia Health Research Institute, group of Cellular Oncology; IKERBASQUE; CIBERfes, Spain.
- 1381 Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Department of Cardiovascular Medicine, Kyoto, Japan.
- 1382 CHU Clermont-Ferrand, Chirurgie Gynécologique, Clermont-Ferrand, France; Université Clermont Auvergne, Institut Pascal, UMR6602, CNRS/UCA/SIGMA, Clermont-Ferrand, France.
- 1383 University of Chieti-Pescara, Department of Medical, Oral, and Biotechnological Science, Chieti, Italy.
- 1384 University of California, Davis, Department of Dermatology, Sacramento, CA, USA.
- 1385 CONACYT - Centro de Investigación Biomédica de Oriente, Instituto Mexicano del Seguro Social, Puebla, Mexico.
- 1386 IRCCS "Casa Sollievo della Sofferenza", Opera di Padre Pio da Pietrelcina, Division of Internal Medicine and Chronobiology Unit, Department of Medical Sciences, San Giovanni Rotondo (FG), Italy.
- 1387 Sapienza University of Rome, Department of Biology and Biotechnology C. Darwin, Rome, Italy.
- 1388 University of Texas Health Science Center at Houston, Center for Stem Cell and Regenerative Disease, Institute of Molecular Medicine (IMM), Houston, TX, USA.
- 1389 Cleveland Clinic, Lerner Research Institute, Department of Inflammation & Immunity, Cleveland, OH, USA.
- 1390 University of Arkansas for Medical Sciences, Fay W. Boozman College of Public Health, Department of Environmental and Occupational Health, Little Rock, AR, USA.
- 1391 University College Cork, Department of Cancer Research, Cork, Ireland.
- 1392 Academic Decency, MeTooSTEM, Nashville, TN, USA.
- 1393 Mayo Clinic, Rochester, MN, USA.
- 1394 Translational Stem Cell Biology Metabolism Program, Research Programs Unit, University of Helsinki, Helsinki, Finland.
- 1395 Department of Anatomy, Faculty of Medicine, Helsinki, Finland.
- 1396 PSL Research University, Equipe labelisée par la Ligue Contre le Cancer, Institut Curie, Inserm, U830, Stress and Cancer laboratory, Paris, France.
- 1397 Sprott Centre for Stem Cell Research, Ottawa Hospital Research Institute, and University of Ottawa, Department of Medicine, Ottawa, ON, Canada.
- 1398 University of Szeged, Department of Medical Microbiology and Immunobiology, Szeged, Hungary.
- 1399 University of Arkansas for Medical Sciences and Central Arkansas Veterans Healthcare System, Little Rock, AR, USA.
- 1400 University of Amsterdam, Medical Biochemistry, Amsterdam UMC, Amsterdam, The Netherlands.
- 1401 Leiden University, Institute of Biology Leiden, Leiden, The Netherlands; The City University of New York, Queens College, The Graduate Center of the City University of New York, Department of Biology, Flushing, NY, USA.
- 1402 UiT The Arctic University of Norway, Department of Pharmacy, Pharmacology Research Group, Tromso, Norway.
- 1403 Childrens' Hospital, Hannover Medical School, Hannover, Germany.
- 1404 Anhui Province Key Laboratory of Medical Physics and Technology, Center of Medical Physics and Technology, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei, Anhui, China; University of Science and Technology of China, Anhui, China.
- 1405 Laboratory of Cell Biology, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil.
- 1406 Indian Institute of Technology Delhi, Kusuma School of Biological Sciences, New Delhi, India.
- 1407 University of Cincinnati, Division of Hematology/Oncology, Cincinnati, OH, USA.
- 1408 University of Pretoria, School of Medicine, Faculty of Health Sciences, Department of Physiology, Pretoria, South Africa.
- 1409 Laboratoire d'Optique et Biosciences, Ecole Polytechnique, CNRS, Inserm, Institut Polytechnique de Paris, Palaiseau, France.
- 1410 University of New Mexico, Clinical and Translational Sciences Center, Albuquerque, NM, USA.
- 1411 Carité-Universitaetsmedizin Berlin, Campus Mitte, Institut für Integrative Neuroanatomie, Berlin, Germany.
- 1412 Ruprecht-Karls University of Heidelberg, Women's Hospital, Department of Gynecological Endocrinology and Fertility Disorders, Heidelberg, Germany.
- 1413 Institut Jean-Pierre Bourgin, INRA, AgroParisTech, CNRS, Université Paris-Saclay, Versailles, France.
- 1414 University of Duisburg-Essen, Centre for Medical Biotechnology, Faculty of Biology, Essen, Germany.
- 1415 Tianjin Medical University, Department of Immunology, School of Basic Medical Sciences, Tianjin, China.
- 1416 INSERM Institute of Metabolic and Cardiovascular Diseases (I2MC), Université de Toulouse, Toulouse, France.
- 1417 Shandong University, School of Life Science, Shandong Provincial Key Laboratory of Animal Cells and Developmental Biology, Qingdao, China.
- 1418 Fondazione IRCCS Casa Sollievo della Sofferenza, Poliambulatorio Giovanni Paolo II, Division of Medical Genetics, San Giovanni Rotondo (FG), Italy.
- 1419 Hirosaki University Graduate School of Medicine, Institute of Brain Science, Department of Neuropathology, Hirosaki, Japan; UCL Queen Square Institute of Neurology, Queen Square Brain Bank for Neurological Disorders, London, UK.
- 1420 National Medicines Institute, Department of Drug Biotechnology and Bioinformatics, Warszawa, Poland.
- 1421 University of Washington School of Medicine, Department of Biochemistry, Seattle, WA, USA.
- 1422 University of Washington, Departments of Medicine, Microbiology and Genome Sciences, Seattle, WA, USA.
- 1423 Sage Therapeutics, Cambridge, MA, USA.
- 1424 European Neuroscience Institute (ENI) - A Joint Initiative of the University Medical Center Göttingen and the Max Planck Society, Göttingen, Germany.
- 1425 Swedish University of Agricultural Sciences and Linnean Center for Plant Biology, Department of Molecular Sciences, Uppsala BioCenter, Uppsala, Sweden; Heidelberg University, Center for Organismal Studies, Neuenheimer Feld, Heidelberg, Germany.
- 1426 Kashan University of Medical Sciences, Research Center for Biochemistry and Nutrition in Metabolic Disease, Kashan, Iran.
- 1427 Tehran University of Medical Sciences, School of Medicine, Department of Medical Immunology, Tehran, Iran.
- 1428 Macquarie University, Faculty of Medicine and Health Sciences, New South Wales, Australia.
- 1429 Indian Institute of Technology Jodhpur, Cellular and Molecular Neurobiology Unit, Jodhpur, Rajasthan, India.
- 1430 Amrita Vishwa Vidyapeetham, School of Biotechnology, Kollam, Kerala, India.
- 1431 University of Nebraska Medical Center, Department of Cellular and Integrative Physiology, Omaha, NE, USA.
- 1432 University of Campania L. Vanvitelli, Department of Precision Medicine, Naples, Italy; Institute of Genetic Research, Biogem scarl, Laboratory of Molecular and Precision Oncology, Ariano Irpino (AV), Italy.
- 1433 University of Campania "Luigi Vanvitelli", Department of Precision Medicine, Naples, Italy.
- 1434 Université Toulouse III, Toulouse, France.
- 1435 Sapporo Medical University, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo, Japan.
- 1436 University of California San Diego, Department of Pharmacology, La Jolla, CA, US.
- 1437 University of Colorado Denver, Department of Medicine, Aurora, CO, USA.
- 1438 Health Sciences University of Hokkaido, College of Rehabilitation Sciences, Department of Physical Therapy, Ishikari-Tobetsu, Hokkaido, Japan.
- 1439 Nagasaki University Graduate School of Biomedical Sciences, Department of Infectious Diseases, Nagasaki, Japan.
- 1440 Tokyo Medical University, Department of Biochemistry, Tokyo, Japan.
- 1441 Aarhus University, Department of Biomedicine, Aarhus, Denmark.
- 1442 Université Côte d'Azur (UCA), Institute for Research on Cancer and Aging of Nice (IRCAN), Centre Antoine Lacassagne, Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), Fédération Hospitalo-Universitaire (FHU) OncoAge, Nice, France.
- 1443 Kerman University of Medical Sciences, Institute of Neuropharmacology, Pharmaceutics Research Center, Kerman, Iran.
- 1444 MVR Cancer Hospital and Research Institute, Molecular Oncology, Cancer Genomics/Proteomics, Kerala, India.
- 1445 University of Kaiserslautern, Department of Biology, Plant Physiology, Kaiserslautern, Germany.
- 1446 International Centre for Genetic Engineering and Biotechnology, New Delhi, India.
- 1447 Department of Experimental Pathology, Institute of Biomedical Research of Barcelona, Spanish Research Council (IIBB-CSIC); IDIBAPS and CIBEREHD, Barcelona, Spain.
- 1448 Washington University in St. Louis, Professor of Obstetrics and Gynecology, St. Louis, MO, USA.
- 1449 Università della Svizzera italiana (USI), Faculty of Biomedical Sciences, Institute for Research in Biomedicine, Bellinzona, Switzerland; School of Life Sciences, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.
- 1450 University "Magna Graecia" of Catanzaro, Department of Health Sciences, Catanzaro, Italy.
- 1451 Aarhus University Hospital, Steno Diabetes Center Aarhus, Aarhus, Denmark; Aarhus University, Department of Clinical Medicine, Research Laboratory for Biochemical Pathology, Aarhus, Denmark.
- 1452 Université de Lyon, ENSL, UCBL, CNRS, LBMC, UMS 3444 Biosciences Lyon Gerland, Lyon, France.
- 1453 Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Científicas, Department of Molecular Biomedicine, Laboratory of Cell Death and Cancer Therapy, Madrid, Spain.
- 1454 Bispebjerg Hospital, Institute of Sports Medicine Copenhagen, Copenhagen,Denmark; UniCamillus, Saint Camillus International University of Health Sciences, Rome, Italy.
- 1455 University of Maryland School of Medicine, Center for Biomedical Engineering and Technology, Baltimore, MD, USA.
- 1456 Università degli Studi di Sassari, Dipartimento di Scienze Biomediche, Sassari, Italy.
- 1457 Centro di Riferimento Oncologico di Aviano (CRO), IRCCS, Immunopathology and Cancer Biomarkers, Aviano, Italy.
- 1458 University of Virginia, Department of Biology, Charlottesville, VA, USA.
- 1459 University of Exeter Medical School, European Centre for Environment & Human Health (ECEHH), Knowledge Spa, Royal Cornwall Hospital, Truro, Cornwall, UK; Plymouth Marine Laboratory (PML), Plymouth, UK; University of Plymouth, School of Biological & Marine Sciences, Plymouth, UK.
- 1460 National Institute of Genetic Engineering and Biotechnology, Institute of Medical Biotechnology, Molecular Medicine Department, Tehran, Iran.
- 1461 University of Pittsburgh, Department of Medicine, Aging Institute, Pittsburgh, PA, USA.
- 1462 Fondazione IRCCS Istituto Neurologico Carlo Besta, Neuromuscular Diseases and Neuroimmunology Unit, Milano, Italy.
- 1463 University of Coimbra, Institute of Physiology, Faculty of Medicine, Coimbra, Portugal and CNC - Center for Neuroscience and Cell Biology, Coimbra, Portugal.
- 1464 European Institute of Oncology (IEO) IRCCS, Department of Experimental Oncology, Milan, Italy.
- 1465 Laboratory of Neurodevelopment, Neurogenetics and Molecular Neurobiology, IRCCS Fondazione Santa Lucia, Rome, Italy.
- 1466 National Autonomous University of Mexico (UNAM), Department of Neurodevelopment and Physiology, Institute of Cellular Physiology, Mexico City, Mexico.
- 1467 Sapienza University of Rome, Department of Anatomy, Histology, Forensic Medicine & Orthopedics, Histology & Medical Embryology Section, Rome, Italy.
- 1468 Ifremer, SG2M-LGPMM, Laboratoire de Génétique et Pathologie des Mollusques Marins, La Tremblade, France.
- 1469 Swansea University Medical School, Molecular Neurobiology group, Swansea, United Kingdom.
- 1470 Normandie University, UNIROUEN, INSERM U1239, DC2N, Rouen, France; Institute for Research and Innovation in Biomedicine (IRIB), Rouen, France.
- 1471 Juntendo University Graduate School of Medicine, Department of Physiology, Tokyo, Japan.
- 1472 University of British Columbia, Department of Ophthalmology and Visual Sciences, Vancouver, British Columbia, Canada.
- 1473 Kindai University, Pharmaceutical Research and Technology Institute, Higashi-Osaka, Osaka, Japan.
- 1474 Saitama University, Department of Regulatory Biology, Saitama, Japan.
- 1475 Pontificia Universidad Católica de Chile, Faculty of Biological Sciences, Department of Physiology, Santiago de Chile, Chile.
- 1476 The University of Texas McGovern Medical School at Houston, Department of Neurology, Houston, TX, USA.
- 1477 Weill Cornell Medicine, Rockefeller University Campus, Department of Pathology and Laboratory Medicine, New York, NY, USA.
- 1478 Max Planck Institute for the Biology of Ageing, Cologne, Germany.
- 1479 Federal University of Ceara, Drug Research and Development Center, Fortaleza, CE, Brazil.
- 1480 Texas Tech University, Department of Nutritional Sciences & Obesity Research Institute, Lubbock, TX, USA.
- 1481 University of Münster, Institute of Medical Microbiology, Münster, Germany.
- 1482 Indian Council of Medical Research - National AIDS Research Institute, Division of Molecular Virology, Pune, MH, India.
- 1483 New York University Medical School, Laura and Isaac Perlmutter Cancer Center, Department of Radiation Oncology, New York, NY, USA.
- 1484 University of Colorado Denver, Children's Hospital Colorado, Morgan Adams Foundation Pediatric Brain Tumor Research Program, Aurora, CO.
- 1485 CNRS Biotechnology and cell signaling, Ecole Supérieure de Biotechnologie de Strasbourg, Strasbourg University/Laboratory of excellence Medalis; University of Strasbourg Institute for Advanced Study, Strasbourg, France.
- 1486 ICREA, Pompeu Fabra University (UPF), Department of Experimental and Health Sciences, Ciberned, Barcelona, Spain; Spanish National Cardiovascular Research Center (CNIC), Madrid, Spain.
- 1487 National Hospital for Paraplagics (Sescam) Research Unit, Molecular Neuroprotection Group, Toledo, Spain.
- 1488 University of Zürich, Institute of Experimental Immunology, Viral Immunobiology, Zürich, Switzerland.
- 1489 King's College London, School of Cardiovascular Medicine and Sciences, London, UK.
- 1490 Old Dominion University, Frank Reidy Research Center for Bioelectrics, Norfolk, VA, USA.
- 1491 Royal College of Surgeons in Ireland, Department of Physiology & Medical Physics, Dublin 2, Ireland.
- 1492 University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada.
- 1493 Genentech, Inc. Department of Cancer Immunology, South San Francisco, CA, USA.
- 1494 University of Helsinki, Faculty of Pharmacy, Division of Pharmacology and Pharmacotherapy/Drug Research Program, Helsinki, Finland.
- 1495 Washington University School of Medicine, Department of Obstetrics and Gynecology, and Department of Pathology and Immunology, St. Louis, MO, USA.
- 1496 University of Rzeszow, Institute of Biology and Biotechnology, Department of Biotechnology, Rzeszow, Poland.
- 1497 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, P.O. Box 19395-5487, Iran.
- 1498 Cornell University, Department of Entomology, Ithaca, NY, USA.
- 1499 Rutgers-New Jersey Medical School, Department of Cell Biology and Molecular Medicine, Newark, NJ, USA.
- 1500 University Hospital of Lausanne, Central Laboratory of Hematology, Lausanne, Switzerland.
- 1501 Kyoto University, Department of Microbiology, Graduate School of Medicine, Kyoto, Japan.
- 1502 Gladstone Institute of Neurological Disease and University of California, San Francisco, Department of Neurology, San Francisco, CA, USA.
- 1503 Tokyo Institute of Technology, School of Life Science and Technology, Yokohama, Japan.
- 1504 University of South Florida, Department of Cell Biology, Microbiology, and Molecular Biology, Tampa, FL, USA.
- 1505 "Sapienza" University of Rome, Department of Clinical and Molecular Medicine, Laboratory affiliated to Istituto Pasteur Italia Fondazione Cenci Bolognetti, Rome, Italy.
- 1506 University of Cambridge, Cancer Research UK Cambridge Institute, Cambridge, UK.
- 1507 Soroka University Medical Center, Institute of Hematology and Ben-Gurion University of the Negev, Faculty of Health Sciences, Department of Clinical Biochemistry and Pharmacology, Beer-Sheva, Israel.
- 1508 Niigata University, Brain Research Institute, Department of Neurosurgery, Niigata, Japan.
- 1509 Nelson Mandela University, Department of Biochemistry and Microbiology, Port Elizabeth, South Africa.
- 1510 Ecole Polytechnique Fédérale de Lausanne (EPFL), Institute of Bioengineering (IBI) and Swiss Institute for Experimental Cancer Research (ISREC), Laboratory of Regenerative Hematopoiesis, Lausanne, Switzerland; Centre Hospitalier Universitaire Vaudois (CHUV), Departments of Oncology and Laboratory Medicine, Hematology Service, Lausanne, Switzerland.
- 1511 University of Arizona, Department of Medicine, Tucson, AZ, USA.
- 1512 Georgia State University, Department of Biology, Atlanta, GA, USA.
- 1513 University of Copenhagen, Novo Nordisk Foundation Center for Basic Metabolic Research, Copenhagen, Denmark.
- 1514 Radboud University Medical Center, Department of Internal Medicine, Nijmegen, The Netherlands.
- 1515 Maastricht University Medical Center, CARIM School for Cardiovascular Diseases, Department of Pathology, Maastricht, The Netherlands.
- 1516 University Hospital Basel, Department of Biomedicine, Ocular Pharmacology and Physiology, Basel, Switzerland.
- 1517 Karolinska Institutet, Department of Women's and Children's Health, Stockholm, Sweden; Astrid Lindgren Children's Hospital, Karolinska University Hospital, Solna, Sweden.
- 1518 King's College London GKT School of Medical Education, London, UK.
- 1519 The University of Sydney, Kolling Institute, Renal Medicine, Sydney, New South Wales, Australia.
- 1520 University of Kansas Medical Center. Department of Pharmacology, Toxicology and Thereapeutics, Kansas City, KS, USA.
- 1521 Trinity College Dublin, Department of Clinical Medicine, Trinity Centre for Health Sciences, Dublin, Ireland.
- 1522 Army Medical University (Third Military Medical University), Daping Hospital, Center of Bone Metabolism and Repair, State Key Laboratory of Trauma, Burns and Combined Injury, Trauma Center, Research Institute of Surgery, Laboratory for the Prevention and Rehabilitation of Military Training Related Injuries, Chongqing, China.
- 1523 University of Ferrara, Department of Chemical and Pharmaceutical Sciences, Ferrara, Italy.
- 1524 National Hospital for Paraplegics (SESCAM), Research Unit, Molecular Neuroprotection Group, Toledo, Spain.
- 1525 East Tennessee State University, Quillen College of Medicine, Center of Excellence for Inflammation, Infectious Diseases, and Immunity, Department of Medicine, Johnson City, TN, USA.
- 1526 ICMR-Vector Control Research Center, Unit of Microbiology and Immunology, Puducherry, India.
- 1527 University of Kansas School of Medicine, Department of Anatomy and Cell Biology, Kansas City, KS, USA.
- 1528 New York University School of Medicine, Departments of Psychiatry and Cell Biology, and the Nathan Kline Institute, Orangeburg, NY, USA.
- 1529 Faculdade de Medicina e Ciências Biomédicas, Universidade do Algarve, Faro, Portugal; ABC-RI, Algarve Biomedical Center Research Institute, Faro, Portugal; Centre for Biomedical Research, Faro, Portugal.
- 1530 Duke University, Department of Biology and Howard Hughes Medical Institute, Durham, NC, USA.
- 1531 University of Split, School of Medicine, Split, Croatia.
- 1532 Doshisha University Graduate School of Brain Science, Laboratory of Structural Neuropathology, Kyoto, Japan.
- 1533 Center for Molecular Biology of Heidelberg University (ZMBH) and German Cancer Research Center (DKFZ), DKFZ-ZMBH Alliance, Heidelberg, Germany.
- 1534 Danish Cancer Society Research Center, Unit for Cell Death and Metabolism, Center for Autophagy, Recycling and Disease, Copenhagen, Denmark; University of Copenhagen, Department of Cellular and Molecular Medicine, Faculty of Health Sciences, Copenhagen N, Denmark.
- 1535 Trinity College Dublin, Trinity Translational Medicine Institute, Dublin, Ireland.
- 1536 David Geffen School of Medicine at UCLA, Department of Microbiology, Immunology, and Molecular Genetics, Los Angeles, CA, USA.
- 1537 University of Messina, Sicily, Italy.
- 1538 Hopp Children's Cancer Center Heidelberg (KiTZ), Heidelberg, Germany; German Cancer Research Center (DKFZ), and German Cancer Consortium (DKTK), Clinical Cooperation Unit Pediatric Oncology, Im Neuenheimer Feld, Heidelberg, Germany.
- 1539 National Institute of Infectious Diseases, Department of Bacteriology I, Toyama, Tokyo, Japan.
- 1540 Université de Paris, Centre de recherche sur l'inflammation, Team Gut Inflammation, Inserm, U1149, CNRS, ERL8252, Paris, France.
- 1541 University of Iceland, Faculty of Medicine, Biomedical center, Reykjavik, Iceland.
- 1542 Hollings Cancer Center, Department of Biochemistry and Molecular Biology, Charleston, SC, USA.
- 1543 Ewha Womans University, Department of Life Science, Immune and Vascular Cell Network Research Center, National Creative Initiatives, Seoul, Korea.
- 1544 Chosun University, School of Medicine, Gwangju, Korea.
- 1545 Yonsei University, Wonju, Korea.
- 1546 Yamaguchi University, Joint Faculty of Veterinary Medicine, Laboratory of Veterinary Pharmacology, Yoshida, Yamaguchi, Japan.
- 1547 Hyogo College of Medicine, Department of Genetics, Hyogo, Japan.
- 1548 University of Perugia, Department of Medicine and Surgery, Perugia, Italy.
- 1549 Stanford University, School of Medicine, Department of Pathology, CA, USA.
- 1550 Osaka University, Laboratory of Mitochondrial Dynamics, Graduate School of Frontier Biosciences, Osaka, Japan.
- 1551 Tokyo Medical and Dental University, Tokyo, Japan.
- 1552 Kyoto University of Advanced Science, Faculty of Bioenvironmental Science, Kameoka, Kyoto, Japan.
- 1553 University of Porto, REQUIMTE/UCIBIO, Faculty of Pharmacy, Porto, Portugal.
- 1554 Amgen Inc., South San Francisco, CA, USA.
- 1555 University of California, Berkeley, Departments of Molecular and Cell Biology and Nutritional Sciences and Toxicology, Berkeley, CA, USA; Chan Zuckerberg Biohub, San Francisco, CA, USA.
- 1556 Rutgers University, Center for Advanced Biotechnology and Medicine, Piscataway, NJ, USA.
- 1557 University of Defense, Faculty of Military Health Sciences, Department of Radiobiology, Hradec Kralove, Czech Republic.
- 1558 The Chinese University of Hong Kong, Lui Che Woo Institute of Innovative Medicine, Centre for Cardiovascular Genomics and Medicine (CCGM), Shatin, Hong Kong, China; The Chinese University of Hong Kong, Faculty of Medicine, Department of Medicine & Therapeutics, Shatin, Hong Kong, China; Hong Kong Hub of Paediatric Excellence (HK HOPE), Hong Kong Children's Hospital, Kowloon, Hong Kong, China; Xiamen University, Xiamen Cardiovascular Hospital, Institute for Translational Medicine, Xiamen, China; Chinese Academy of Sciences, Kunming Institute of Zoology - The Chinese University of Hong Kong (KIZ-CUHK) Joint Laboratory of Bioresources and Molecular Research of Common Diseases, Kunming, Yunnan, China.
- 1559 The University of Illinois at Chicago College of Medicine, Department of Pharmacology, Chicago, IL, USA.
- 1560 Pontificia Universidad Católica de Chile, Escuela de Medicina and Centro Interdisciplinario de Neurociencias, Facultad de Medicina, Departamento de Neurología, Santiago, Chile.
- 1561 University of Pennsylvania Perelman School of Medicine and Division of Neurology, The Children's Hospital of Philadelphia, Department of Neurology, Philadelphia, PA, USA.
- 1562 Suez Canal University, Faculty of Veterinary Medicine, Ismailia, Egypt.
- 1563 University of Wisconsin-Madison, Center for Quantitative Cell Imaging and Department of Botany, Madison, WI, USA.
- 1564 German Institute of Human Nutrition Potsdam-Rehbruecke, Department of Molecular Toxicology, Nuthetal, Germany.
- 1565 Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy; Institute of Molecular Bioimaging and Physiology (IBFM), CNR, Milan, Italy.
- 1566 University of Southern California, Keck School of Medicine, Department of Molecular Microbiology and Immunology, Los Angeles, CA, USA.
- 1567 University Medical Center Göttingen, Center for Biostructural Imaging of Neurodegeneration, Department of Experimental Neurodegeneration, Göttingen, Germany; Max Planck Institute for Experimental Medicine, Göttingen, Germany; Newcastle University, The Medical School, Institute of Neuroscience, Newcastle upon Tyne, UK.
- 1568 Oslo University Hospital and University of Oslo, Department of Pathology, Tumor Immunology Lab, Oslo, Norway.
- 1569 Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Medical Biology Department, Istanbul, Turkey.
- 1570 Texas Biomedical Research Institute, Host-Pathogen Interactions Program, San Antonio, TX, USA.
- 1571 Baylor College of Medicine, Department of Molecular and Human Genetics, Houston, TX, USA.
- 1572 University of Naples Federico II, Department of Molecular Medicine and Medical Biotechnology, Naples, Italy.
- 1573 Université de Paris, Centre de Recherche des Cordeliers, Paris, France.
- 1574 Fondazione IRCCS Ospedale San Raffaele, Division of Neuroscience, Milan, Italy.
- 1575 University of São Paulo, Department of Parasitology, São Paulo, Brazil.
- 1576 University of Rome Tor Vergata, Department of Clinical Sciences and Translational Medicine, Rome, Italy.
- 1577 Goethe University Frankfurt, Buchmann Institute for Molecular Life Sciences (BMLS), Frankfurt am Main, Germany.
- 1578 Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, China.
- 1579 Affiliated Hospital of GuangDong Medical University, Key Laboratory of Prevention and Management of Chronic Kidney Disease of Zhanjiang City, Zhanjiang, Guangdong, China.
- 1580 Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
- 1581 National Center of Biomedical Analysis, Beijing, China.
- 1582 The Children's Hospital of Philadelphia, Center for Applied Genomics, Philadelphia, PA, USA.
- 1583 University of Pittsburgh Medical Center, Department of Pediatrics, Pittsburgh, PA, USA.
- 1584 University of Alberta, Department of Biochemistry, Edmonton, Canada.
- 1585 University of Salento, Department of Biological and Environmental Sciences and Technologies (Di.S.Te.B.A.), Lecce, Italy.
- 1586 Danish Cancer Society Research Center, Computational Biology Laboratory, Copenhagen, Denmark.
- 1587 Max Perutz Labs, University of Vienna, Vienna Biocenter (VBC), Vienna, Austria.
- 1588 Quadram Institute Bioscience, Department of Gut, Microbes and Health, Norwich, UK.
- 1589 Waksman Institute, Department of Genetics, Rutgers University, Piscataway, NJ, USA.
- 1590 Dong-A University Medical School, Department of Molecular Neuroscience, Busan, Korea.
- 1591 Medical College of Wisconsin, Department of Biochemistry, Milwaukee, WI, USA.
- 1592 Incheon National University, Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon, Korea.
- 1593 Yonsei University, Division of Biological Science and Technology, Wonju, Korea.
- 1594 Chonnam National University, The Future Life & Society Research Center, Gwangju, Korea.
- 1595 Chonbuk National University, Department of VeterinaryMedicine, Iksan, Korea.
- 1596 Ben Gurion University of the Negev, Department of Chemistry, Be'er Sheva, Israel.
- 1597 KU Leuven, Department of Cellular and Molecular Medicine & Leuven Kanker Instituut, Leuven, Belgium.
- 1598 Sanofi, Vitry Sur Seine, France.
- 1599 Mayo Clinic, Department of Physiology and Biomedical Engineering, Rochester, MN, USA.
- 1600 VA San Diego Healthcare Systems and University of California, San Diego, Department of Anesthesiology, La Jolla, CA, USA.
- 1601 Internal Medicine 1, Brandenburg Hospital, Faculty of Health Sciences, Joint Faculty of the Brandenburg University of Technology Cottbus Senftenberg, the Brandenburg Medical School Theodor Fontane and the University of Potsdam, Brandenburg, Germany.
- 1602 IRCM, Institut de Recherche en Cancérologie de Montpellier, Tumor Microenvironment and Resistance to Treatment Laboratory, INSERM U1194, Université de Montpellier, Institut régional du Cancer de Montpellier, Montpellier, France.
- 1603 Universidad Nacional Autónoma de México, Instituto de Biotecnología, Departamento de Medicina Molecular y Bioprocesos, Laboratorio de Neuroinmunobiología, Cuernavaca, Morelos, México.
- 1604 Universidad de Chile, School of Medicine, Instituto de Ciencias Biomédicas, Santiago de Chile, Chile.
- 1605 Friedrich-Loeffler-Institut, Insitute of Immunology, Greifswald - Insel Riem, Germany.
- 1606 The Fourth Military Medical University, National Key Discipline of Cell Biology, Department of Physiology and Pathophysiology, Xi'an, Shaanxi, China.
- 1607 Weizmann Institute of Science, Department of Plant and Environmental Sciences, Rehovot, Israel.
- 1608 Centro de Investigaciones Biológicas CSIC, Department of Cellular and Molecular Biology, Madrid, Spain.
- 1609 Sun Yat-Sen University, Sun Yat-sen Memorial Hospital, Department of Neurology, Guangzhou City, Guangdong, China.
- 1610 University of Padova, Department of Biomedical Sciences, Padova, Italy.
- 1611 Fondazione G. Pascale, Istituto Nazionale Tumori IRCCS, Cell Biology and Biotherapy Unit, Naples, Italy.
- 1612 University of Coimbra, CNC - Center for Neuroscience and Cell Biology & Faculty of Medicine & CIBB - Center for Innovative Biomedicine and Biotechnology, Coimbra, Portugal.
- 1613 Universidade Federal de São Paulo, Paulista School of Medicine, Departament of Pharmacology, São Paulo, Brazil.
- 1614 São Paulo State University, College of Agronomy Sciences, Department of Bioprocesses and Biotechnology, Center for Evaluation of Environmental Impact on Human Health (TOXICAM), Botucatu, SP, Brazil.
- 1615 University of Melbourne, Florey Institute of Neuroscience and Mental Health, Melbourne, VIC, Australia.
- 1616 University of Granada, Faculty of Pharmacy, Department of Physical Chemistry, Granada, Spain.
- 1617 University of Nebraska Medical Center, Department of Pharmacology and Experimental Neuroscience, Omaha, NE, USA.
- 1618 State University of New York, Upstate Medical University, Departments of Medicine, Microbiology and Immunology, Biochemistry and Molecular Biology, Syracuse, NY, USA.
- 1619 Università degli Studi di Milano, Department of Biomedical and Clinical Sciences "L. Sacco", Milan, Italy.
- 1620 University of Calabria, Department of Biology, Ecology and Earth Sciences, Centre for Microscopy and Microanalysis (CM2), Rende (CS), Calabria, Italy.
- 1621 Baruch S. Blumberg Institute, Pennsylvania Cancer and Regenerative Medicine Research Center, Wynnewood, PA, USA; Xavier University School of Medicine, Woodbury, NY, USA.
- 1622 National Jewish Health and the University of Colorado, Denver, CO, USA.
- 1623 University of Oslo, Oslo University Hospital and Institute for Clinical Medicine, Department of Ophthalmology, Center for Eye Research, Oslo, Norway.
- 1624 Health and Medical University, Potsdam, Germany.
- 1625 Ulm University, Institute of Biochemistry and Molecular Biology, Faculty of Medicine, Ulm, Germany.
- 1626 Washington University, Division of Infectious Diseases, Department of Medicine and Molecular Microbiology, St. Louis, MO, USA.
- 1627 Third Military Medical University, Department of Occupational Health, Chongqing, China.
- 1628 Virginia Polytechnic Institute and State University, School of Neuroscience, Blacksburg, VA, USA.
- 1629 Aix Marseille Université, CNRS, INSERM, CIML, 13288 Marseille cedex 9, France; Institute for Research in Biomedicine (iBiMED) and Ilidio Pinho Foundation, Department of Medical Sciences, University of Aveiro, 3810-193 Aveiro, Portugal; Shanghai Institute of Immunology, Department of Microbiology and Immunology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, PR China.
- 1630 Karolinska Institutet, Department of Biosciences and Nutrition, Huddinge, Sweden.
- 1631 Consejo Superior de Investigaciones Científicas (CSIC), Instituto de Biología Molecular y Celular del Cáncer (IBMCC), Centro de Investigación del Cáncer (CSIC-Universidad de Salamanca), Universidad de Salamanca, Campus Unamuno, Salamanca. Spain.
- 1632 Tel Aviv University, School of Neurobiology, Biochemistry and Biophysics, Tel Aviv, Israel.
- 1633 The University of Texas MD Anderson Cancer Center, Department of Leukemia, Houston, TX, USA.
- 1634 Radboud University Medical Center, Nijmegen, The Netherlands.
- 1635 Ruhr-Universität Bochum, Medizinische Fakultät, Biochemie Intrazellulärer Transportprozesse, Bochum, Germany.
- 1636 Achucarro Basque Center for Neuroscience, Department of Pharmacology, University of the Basque Country EHU/UPV, Leioa, Bizkaia, Spain.
- 1637 University of Cologne, Center for Biochemistry, Cologne, Germany.
- 1638 Moscow State University, A.N.Belozersky Institute of Physico-Chemical Biology, Laboratory of Structure and function of mitochondria, Moscow, Russia.
- 1639 UCL Queen Square Institute of Neurology, London, UK.
- 1640 Monash Biomedicine Discovery Institute and Monash University, Department of Anatomy and Developmental Biology, Melbourne, Victoria, Australia.
- 1641 Georg-August University Göttingen, Institute of Microbiology and Genetics, Department of Genetics of Eukaryotic Microorganisms, Göttingen, Germany.
- 1642 Buchmann Institute & Institute of Biochemistry II, Medical Faculty, Goethe University, Frankfurt, Germany.
- 1643 Cancer Research Center of Toulouse, UMR 1037 Inserm-university of Toulouse, Toulouse, France.
- 1644 Mahidol University, Department of Microbiology, Faculty of Science, Ratchathewi, Bangkok, Thailand.
- 1645 Erasmus University Medical Center, Department of Epidemiology; Division of Pharmacology, Department of Internal Medicine, Rotterdam, The Netherlands.
- 1646 The Hospital for Sick Children, Translational Medicine Program, Toronto, Canada.
- 1647 St. Jude Children's Research Hospital, Department of Cell and Molecular Biology, Memphis, TN, USA.
- 1648 University of Oxford, Nuffield Department of Women's and Reproductive Health, Oxford, UK.
- 1649 University of California, Davis, Department of Molecular and Cellular Biology, Davis, CA, USA.
- 1650 University of Iowa, Department of Biology, Aging Mind and Brain Initiative, Iowa City, IA, USA.
- 1651 Jagiellonian University, Department of Evolutionary Immunology, Faculty of Biology, Krakow, Poland.
- 1652 Temple University, Alzheimer's Center at Temple, Philadelphia, PA, USA.
- 1653 Eberhard Karls University Tübingen, Interfaculty Institute of Cell Biology, Tübingen, Germany.
- 1654 University of Cyprus, Department of Biological Sciences, Bioinformatics Research Laboratory, Nicosia, Cyprus.
- 1655 Lifelong Health, South Australian Health & Medical Research Institute, North Terrace, Adelaide, Australia.
- 1656 University of Wisconsin-Madison, Department of Medicine and Neuroscience, Madison, WI, USA.
- 1657 Dalhousie University, Department of Biochemistry and Molecular Biology, Dalhousie Medicine New Brunswick, Saint John, NB, Canada.
- 1658 National Center for Cell Science, Pune, India.
- 1659 CURML, University Center of Legal Medicine, Lausanne University Hospital, Lausanne, Switzerland.
- 1660 Chinese Academy of Sciences, Kunming Institute of Zoology, Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences, Kunming, Yunnan, China.
- 1661 Lanzhou University, School of Life Sciences, Lanzhou, China.
- 1662 Zhejiang University, College of Medicine, The First Affiliated Hospital, Malignant Lymphoma Diagnosis and Therapy Center, Hangzhou, Zhejiang, China.
- 1663 China Pharmaceutical University, School of Basic Medicine and Clinical Pharmacy, State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Carcinogenesis and Intervention, Nanjing, China.
- 1664 Sanofi, Biologics Research, 49 New York Ave, Framingham, MA, USA.
- 1665 University of Waterloo, Department of Kinesiology, Waterloo, Ontario, Canada.
- 1666 Instituto de Investigaciones Biomedicas en Retrovirus y Sida, Universidad de Buenos Aires-CONICET, Argentina.
- 1667 University of Pittsburgh, Department of Pathology; University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
- 1668 Dartmouth College, Department of Chemistry, Hanover, NH, USA.
- 1669 Boston University, Department of Pathology and Laboratory Medicine, Boston, MA, USA.
- 1670 Tehran University of Medical Sciences, Cancer Biology Research Center, Tehran, Iran.
- 1671 Penn State University College of Medicine, Department of Cellular and Molecular Physiology, Hershey, PA, USA.
- 1672 University of Alabama at Birmingham, Cardiac Aging & Redox Signaling Laboratory, Molecular and Cellular Pathology, Department of Pathology, Birmingham, AL, USA.
- 1673 Johann Wolfgang Goethe-University, Institute of Anatomy III- 'Cellular and Molecular Anatomy', Frankfurt, Germany.
- 1674 University of Birmingham, Institute of Metabolism and Systems Research, Birmingham, UK.
- 1675 MRC Laboratory of Molecular Biology, Division of Protein and Nucleic Acid Chemistry, Cambridge, UK.
- 1676 Indian Institute of Science, Department of Biochemistry, Bangalore, India.
- 1677 School of Medicine, the Southern University of Science and Technology, Shenzhen, China.
- 1678 Baylor College of Medicine, Department of Medicine, Houston, TX, USA.
- 1679 The University of Kansas Cancer Center, Division of Hematologic Malignancies and Cellular Therapeutics, Kansas City, KS, USA.
- 1680 All India Institute of Medical Sciences, Department of Biotechnology, New Delhi, India.
- 1681 University of Southampton, Clinical and Experimental Sciences, Faculty of Medicine, UK.
- 1682 Johns Hopkins University School of Medicine, Department of Otolaryngology/Head and Neck Surgery, Head and Neck Cancer Research Division, Baltimore, MD, USA.
- 1683 University of South Carolina School of Medicine, Department of Pathology, Microbiology, and Immunology, Columbia, SC, USA.
- 1684 Washington University School of Medicine, Department of Medicine, Cardiovascular Division,l and John Cochran VA Medical Center, St. Louis, MO, USA.
- 1685 Johns Hopkins University, Bloomberg School of Public Health, Biochemistry and Molecular Biology Department, Baltimore, MD, USA.
- 1686 Johannes Gutenberg University-Mainz, Institute of Developmental Biology and Neurobiology, Mainz, Germany.
- 1687 Heinrich Heine University Düsseldorf, Medical faculty, Institute of Biochemistry and Molecular Biology I, Düsseldorf, Germany.
- 1688 The University of Texas MD Anderson Cancer Center, Institute for Applied Cancer Science (IACS), Translational Research to Advance Therapeutics and Innovation in Oncology (TRACTION), Houston, TX, USA.
- 1689 Max Planck Institute of Psychiatry, Translational Research in Psychiatry, Munich, Germany.
- 1690 University of Vienna, Cell Imaging and Ultrastructure Research (CIUS), Vienna, Austria.
- 1691 Karolinska Institutet, Department of Laboratory Medicine, Division of Clinical Microbiology, Stockholm, Sweden.
- 1692 Wright State University, Department of Biochemistry & Molecular Biology, Dayton, OH. USA.
- 1693 University of Wyoming, College of Health Sciences, Laramie, WY, USA.
- 1694 Qingdao Agricultural University, College of Plant Health and Medicine, Qingdao, Shandong, China.
- 1695 Ifremer, RBE, Département Ressources, La Tremblade, France.
- 1696 University of California, Los Angeles, David Geffen School of Medicine, Department of Human Genetics, Los Angeles, CA, USA.
- 1697 Botucatu Medical School, São Paulo State University, Botucatu, SP, Brazil.
- 1698 Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France.
- 1699 The Ohio State University, College of Veterinary Medicine, Department of Veterinary Biosciences, Columbus, OH, USA.
- 1700 Thomas Jefferson University, Department of Orthopaedic Surgery and Graduate Program in Cell Biology and Regenerative Medicine, Philadelphia, PA, USA.
- 1701 Josep Carreras Leukaemia Research Institute, Barcelona, Spain.
- 1702 Democritus University of Thrace, First Department of Internal Medicine & Laboratory of Molecular Hematology, Alexandroupolis, Greece.
- 1703 Redox Signaling and Oxidative Stress group, Danish Cancer Society Research Center, Copenhagen, Denmark.
- 1704 University of Padua, Department of Biomedical Sciences, Padua, Italy.
- 1705 Middlesex University, Department of Natural Sciences, London, UK.
- 1706 INSERM U1065, Centre Méditerranéen de Médecine Moléculaire (C3M), Nice, France; Université de Nice Sophia Antipolis, UFR de Médecine, Nice, France.
- 1707 Baylor College of Medicine, Department of Molecular Physiology & Biophysics, Houston, TX, USA.
- 1708 Federal University of ABC (UFABC), Center for Natural and Human Sciences (CCNH), Santo André, SP, Brazil.
- 1709 University of Campinas (UNICAMP), School of Applied Science, Department of Sport Sciences, Limeira, Brazil.
- 1710 University of Barcelona, School of Medicine, Unit of Anatomy, Department of Pathology and Experimental Therapeutics, Barcelona, Spain; L'Hospitalet de Llobregat, Institut d'Investigació Biomèdica de Bellvitge -IDIBELL, Molecular Mechanisms and Experimental Therapy in Oncology Program (oncobell), Barcelona, Spain.
- 1711 Kansas City University of Medicine and Biosciences, Department of Basic Science, Kansas City, MO, USA.
- 1712 Goethe University Frankfurt, Institute of Pharmaceutical Chemistry, Frankfurt am Main, Germany.
- 1713 University of Gdansk, Faculty of Chemistry, Gdansk, Poland.
- 1714 Department of Medicine and Surgery, University of Perugia, Perugia Italy.
- 1715 Università degli Studi di Catania, Dipartimento di Chirurgia Generale e Specialità Medico-Chirurgiche, Catania, Italy.
- 1716 CONICET-Universidad Nacional de Cuyo, Instituto de Histología y Embriología de Mendoza, Mendoza, Argentina.
- 1717 INSERM U1151, Institut Necker Enfants-Malades (INEM), Team 8, Université Paris Descartes-Sorbonne-Paris Cité, Paris, France.
- 1718 Naples University, Department of Veterinary Medicine and Animal Productions, Naples, Italy.
- 1719 University of Würzburg, Institute of Pathology, Würzburg, Germany.
- 1720 Kiel University and University Hospital Schleswig-Holstein, Campus Kiel, Institute of Clinical Molecular Biology, Kiel, SH, Germany.
- 1721 Georgetown University, Department of Biology, Washington, DC, USA.
- 1722 Columbia University, Department of Pathology and Cell Biology, New York, NY, USA.
- 1723 University of Illinois, Department of Anesthesiology, Chicago, IL, USA.
- 1724 Normandy University, UNICAEN, INSERM, UMR-S U1237, Physiopathology and Imaging of Neurological Disorders (PhIND), Institute Blood and Brain @Caen-Normandie (BB@C), GIP Cyceron, Caen, FRANCE.
- 1725 University of Sherbrooke, Division of Rheumatology, Department of Medicine, Sherbrooke, QC, Canada.
- 1726 Section on Eukaryotic DNA Replication, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD,USA.
- 1727 University of Seville. School of Pharmacy, Instituto de Biomedicina de Sevilla. Sevilla, Spain.
- 1728 University of Cambridge, Cambridge Institute for Medical Research, UK Dementia Research Institute and Department of Medical Genetics, Cambridge, UK.
- 1729 Lomonosov Moscow State University, Chemistry Department, Moscow, Russia.
- 1730 University Medical Center Eppendorf, Institute for Medical Microbiology, Hamburg, Germany.
- 1731 Charles University, Faculty of Medicine in Hradec Kralove, Department of Medical Biology and Genetics, Hradec Kralove, Czech Republic.
- 1732 Mannheim University of Applied Sciences, Faculty of Biotechnology, Mannheim, Germany.
- 1733 Fondazione IRCCS Istituto Neurologico Carlo Besta, Neuromuscular Disease and Immunology Unit, Milan, Italy; University of Brescia, Unit of Biology and Genetics, Department of Molecular and Translation Medicine, Brescia, Italy.
- 1734 Australian Regenerative Medicine Institute, Monash University, Clayton, Victoria, Australia.
- 1735 University of Ottawa, Department of Cellular and Molecular Medicine, Ottawa, ON, Canada.
- 1736 National Research Council, Institute of Food Sciences, Avellino, Italy.
- 1737 Stazione Zoologica "Anton Dohrn", Napoli, Italy.
- 1738 University of Calabria, Department of Pharmacy, Health and Nutritional Sciences, Cosenza, Italy.
- 1739 N.N. Blokhin National medical research center of oncology, Department of biomarkers and mechanisms of tumor angiogenesis, Moscow, Russia.
- 1740 Cancer Research UK Beatson Institute, Glasgow, UK.
- 1741 University of Seoul, Department of Life Science, Seoul, Korea.
- 1742 University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
- 1743 Concordia University, Department of Biology, Montreal, Quebec, Canada; McGill University, Department of Anatomy and Cell Biology, Montreal, Quebec, Canada.
- 1744 Ernst-Ruska Centre for Microscopy and Spectroscopy with Electrons (ER-C-3/StructuralBiology), Forschungszentrum Jülich, Jülich, Germany.
- 1745 Saitama University, Graduate School of Science and Engineering, Saitama, Japan.
- 1746 Rutgers New Jersey Medical School, Department of Cell Biology and Molecular Medicine, Newark, NJ, USA.
- 1747 Oregon State University, College of Pharmacy, Department of Pharmaceutical Sciences, Portland, OR, USA.
- 1748 Mashhad University of Medical Sciences, Pharmaceutical Technology Institute, Biotechnology Research Center, Mashhad, Iran.
- 1749 Boston Children's Hospital and Harvard Medical School, Boston, MA, USA.
- 1750 University of South Carolina, Department of Drug Discovery and Biomedical Sciences, Columbia, SC, USA.
- 1751 University of Sydney, Northern Clinical School, Kolling Institute of Medical Research, Sydney, NSW, Australia.
- 1752 Institute for Research in Biomedicine (IRB Barcelona), Cellular Plasticity and Disease Group, Barcelona, Spain.
- 1753 University of Toyama, Sugitani, Toyama, Japan.
- 1754 Kyoto University, Graduate School of Agriculture, Division of Applied Life Sciences, Kyoto, Japan.
- 1755 The University of Tokyo, Graduate School of Medicine, Department of Biochemistry and Molecular Biology, Tokyo, Japan.
- 1756 University of Helsinki and Helsinki University Hospital, Department of Virology, Helsinki, Finland.
- 1757 Florida State University, Department of Nutrition, Food and Exercise Sciences, Tallahassee, FL, USA.
- 1758 Institut Hospital del Mar d'Investigacions Mèdiques (IMIM), Research Group in Critical Disorders (GREPAC), Barcelona, Spain.
- 1759 Agricultural Biotechnology Research Institute of Iran (ABRII), Department of Systems Biology, Karaj, Iran.
- 1760 University of Minho, School of Medicine, Life and Health Sciences Research Institute (ICVS), Braga, Portugal; ICVS/3B's - PT Government Associate Laboratory, Braga/Guimarães, Portugal.
- 1761 CABD/CSIC/Universidad Pablo de Olavide, Departamento de Fisiología, Anatomía y Biología Celular, Sevilla, Spain.
- 1762 University of Malaga, Department of Molecular Biology and Biochemistry, Malaga, Spain.
- 1763 Imperial College London, Section of Paediatric Infectious Disease & Virology, London, UK.
- 1764 INRS-Institut Armand-Frappier, Laval, QC, Canada.
- 1765 Weill-Cornell, Englander Institute of Precision Medicine, Department of Radiation Oncology, New York, NY, USA.
- 1766 Centre for Innovative Biomedicine and Biotechnology, University of Coimbra, Coimbra, Portugal.
- 1767 University of Camerino, School of Pharmacy, Experimental Medicine Section, Camerino, Italy.
- 1768 University of Glasgow, Institute of Molecular, Cell and Systems Biology, Glasgow, Scotland, United Kingdom.
- 1769 Instituto de Biomedicina de Valencia, CSIC, Valencia, Spain.
- 1770 Jawaharlal Nehru University, School of Life Sciences, New Delhi, India.
- 1771 Institute for Stem Cell Science & Regenerative Medicine (inStem), Bangalore, Karnataka, India.
- 1772 University of Maryland School of Medicine, Department of Anesthesiology, Shock, Trauma and Anesthesiology Research Center, Baltimore, MD, USA.
- 1773 University of Birmingham, Institute of Biomedical Research, Institute of Cancer and Genomic Sciences, College of Medical and Dental Sciences, Edgbaston, Birmingham, UK.
- 1774 Health Research Institute Germans Trias i Pujol, Innate Immunity Group, Badalona, Barcelona, Spain.
- 1775 University of Delhi South Campus, Department of Genetics, New Delhi, India.
- 1776 Folkhälsan Research Center, Helsinki, Finland.
- 1777 Mayo Clinic, Department of Molecular Medicine, Rochester, MN, USA.
- 1778 National Institute of Mental Health and Neurosciences, Department of Neurochemistry, Bengaluru, Karnataka, India.
- 1779 Duke University, Department of Molecular Genetics and Microbiology, Durham, NC, USA.
- 1780 Goethe University, Institute of Pharmacology and Toxicology, Frankfurt, Germany.
- 1781 University of Notre Dame, Department of Biological Sciences, Notre Dame, IN ,USA.
- 1782 UCL Queen Square Institute of Neurology, Department of Clinical and Movement Neurosciences, London, UK.
- 1783 University Hospital and University of Zürich, Department of Gastroenterology and Hepatology, Zürich, Switzerland.
- 1784 University of Calgary, Department of Comparative Biology & Experimental Medicine, Calgary, AB, Canada.
- 1785 Department of Biochemistry and Molecular Biology, Institute for Human Infection and Immunity (IHII), University of Texas Medical Branch at Galveston, Galveston, TX, USA.
- 1786 Amsterdam University Medical Centers location VUmc, Department of Clinical Genetics, Amsterdam, The Netherlands.
- 1787 University of Kaiserslautern, Phytopathology, Kaiserslautern, Germany.
- 1788 IRCCS San Raffaele Scientific Institute, Division of Genetics and Cell Biology, Milan, Italy.
- 1789 Centro di Riferimento Oncologico, CRO-IRCCS, Molecular Oncology Unit, Aviano, Italy.
- 1790 Medical University of Graz, Gottfried Schatz research center, Graz, Austria.
- 1791 University Grenoble Alpes and Inserm U1055, Laboratory of Fundamental and Applied Bioenergetics (LBFA), Grenoble, France.
- 1792 Medical University of Innsbruck, Institute for Cell Biology, Innsbruck, Austria.
- 1793 Hannover Medical School, Department of Nephrology and Hypertension, Hannover, Germany.
- 1794 National Institutes of Health, National Institute of Allergy and Infectious Disease, Vaccine Research Center, Bethesda, MD, USA.
- 1795 Ruhr-University Bochum, Department of Molecular Immunology, Bochum, Germany.
- 1796 German Center for Neurodegenerative Diseases, DZNE, Bonn, Germany and Department of Neurodegenerative Diseases and Geriatric Psychiatry, University Bonn, Bonn, Germany.
- 1797 Reseach Center Borstel, Leibniz Lung Center, Priority Research Area Infections, Junior Research Group Coinfection, Borstel, Germany.
- 1798 Johannes Kepler University Linz, Institute of Biophysics, Linz, Austria.
- 1799 Instituto de Investigaciones en Ingeniería Genética y Biología Molecular Dr. Héctor N. Torres, Laboratorio de Señalización y Mecanismos Adaptativos en Tripanosomátidos, Buenos Aires, Argentina; Universidad de Buenos Aires, Departamento de Fisiología, Biología Molecular y Celular, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina.
- 1800 Instituto de Investigaciones en Ingeniería Genética y Biología Molecular Dr. Héctor N. Torres, Laboratorio de señalización y mecanismos adaptativos en Tripanosomátidos, Buenos Aires, Argentina; Universidad de Buenos Aires, Departamento de Química Biológica, Facultad de Ciencias Exactas y Naturales, Buenos Aires, Argentina.
- 1801 Mayo Clinic, Department of Biochemistry and Molecular Biology, Division of Gastroenterology and Hepatology, Rochester, MN, USA.
- 1802 Technische Universität Dresden, Institute for Physiological Chemistry, Dresden, Germany.
- 1803 University of Wuerzburg, Department of Vegetative Physiology, Wuerzburg, Germany.
- 1804 University of Natural Resources and Life Sciences, Department of Applied Genetics and Cell Biology, Vienna, Austria.
- 1805 Mayo Clinic, Division of Gastroenterology and Hepatology, Rochester, MN, USA.
- 1806 University of Luxembourg, Luxembourg Centre for Systems Biomedicine (LCSB), Esch-sur-Alzette, Luxembourg.
- 1807 Forschungszentrum Jülich, Institute of Biological Information Processing, IBI-7 (Structural Biochemistry), Jülich, Germany.
- 1808 University of Campania L. Vanvitelli, Dipartimento di Scienze Mediche Traslazionali, Naples, Italy.
- 1809 Department of Medical and Surgical Sciences and Biotechnologies, Sapienza University of Rome, Italy; IRCCS Neuromed, Pozzilli (IS), Italy.
- 1810 Rutgers Biomedical and Health Sciences, Rutgers University, Robert Wood Johnson Medical School, Department of Pharmacology, New Brunswick , NJ, USA.
- 1811 National Research Council, Institute of Molecular Genetics, Pavia, Italy.
- 1812 Helmholtz Centre for Infection Research, Braunschweig, Germany.
- 1813 Albert Einstein College of Medicine, Department of Developmental and Molecular Biology, Bronx, NY, USA; Albert Einstein College of Medicine, Institute for Aging Studies, Bronx, NY, USA.
- 1814 Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Barcelona, Spain; Departament de Bioquímica i Biomedicina Molecular, Facultat de Biologia, Universitat de Barcelona, Barcelona, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid, Spain.
- 1815 Rice University, Department of Bioengineering, Houston, TX, USA.
- 1816 University of Illinois at Chicago, College of Medicine, Department of Biochemistry and Molecular Genetics, Chicago, IL, USA.
- 1817 University of Oslo, Centre for Molecular Medicine Norway (NCMM), Oslo, Norway.
- 1818 Université de Pau et des Pays de l'Adour, E2S UPPA, INRAE, UMR1419 Nutrition Métabolisme et Aquaculture, F-64310 Saint-Pée-sur-Nivelle, France.
- 1819 Cedars-Sinai Medical Center, Department of Medicine, Division of Digestive and Liver Diseases, Los Angeles, CA, USA.
- 1820 Pennsylvania State University, Department of Biochemistry and Molecular Biology, University Park, PA, USA.
- 1821 Alpert Medical School of Brown University/Rhode Island Hospital, Division of Cardiothoracic Surgery, Providence, RI, USA.
- 1822 Iowa State University, Animal Science Department, Ames, IA, USA.
- 1823 Dokuz Eylul University, Izmir International Biomedicine and Genome Institute, Izmir, Turkey.
- 1824 University of Alberta, Department of Laboratory Medicine and Pathology, Edmonton, AB, Canada.
- 1825 University of Rochester Medical Center, Microbiology and Immunology, Rochester, NY, USA.
- 1826 Johns Hopkins University School of Medicine, Department of Cell Biology, Baltimore, MD, USA.
- 1827 Indian Institute of Science, Department of Microbiology and Cell Biology, Bangalore, KA, India.
- 1828 Shenzhen University Health Science Center, School of Dentistry, Shenzhen, Guangdong Province, China.
- 1829 University of Alabama at Birmingham, Department of Pharmacology & Toxicology, Birmingham, AL, USA.
- 1830 University of Washington, Department of Medicine, Seattle, WA, USA; VA Puget Sound Health Care System, Seattle, WA, USA.
- 1831 Soochow University, Institutes for Translational Medicine, Suzhou, Jiangsu, China.
- 1832 Freie Universitaet Berlin, Department of Veterinary Medicine, Institute of Veterinary Biochemistry, Berlin, Germany.
- 1833 Johns Hopkins University, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, USA.
- 1834 University of Pennsylvania, Abramson Cancer Center, Department of Medicine, PA, USA.
- 1835 Division of Stem Cell and Gene Therapy Research, Institute of Nuclear Medicine & Allied Sciences (INMAS), Defence Research and Development Organization (DRDO), Timar Pur, New Delhi, India.
- 1836 Thomas Jefferson University, Center for Translational Medicine, Philadelphia, PA, USA.
- 1837 Women and Infants Hospital of Rhode Island-Warren Alpert Medical School of Brown University, Department of Pediatrics, Providence, RI, USA.
- 1838 University of Macau, Faculty of Health Sciences, Macau, China.
- 1839 The University of Hong Kong, School of Chinese Medicine, Hong Kong, China.
- 1840 Nanjing Agricultural University, College of Animal Science and Technology, Nanjing, Jiangsu, China.
- 1841 Shanghai Jiao Tong University School of Medicine, Department of Hypertension, Ruijin Hospital, Shanghai, China.
- 1842 Total Toxicology Labs, Southfield, MI, USA.
- 1843 Soochow University, College of Pharmaceutical Sciences, Department of Pharmacology and Laboratory of Aging and Nervous Diseases, Suzhou, China.
- 1844 Fralin Biomedical Research Institute at VTC, Roanoke, VA, USA.
- 1845 Indiana University School of Medicine, Department of Pediatrics, Indianapolis, IN, USA.
- 1846 Van Andel Institute, Center for Epigenetics, Grand Rapids, MI, USA.
- 1847 Fudan University, Zhongshan Hospital, Liver Cancer Institute, Department of Liver Surgery & Transplantation, Shanghai, China.
- 1848 Juntendo University Graduate School of Medicine, Department of Treatment and Research in Multiple Sclerosis and Neuro-intractable Disease, Bunkyo-ku, Tokyo, Japan.
- 1849 National Chung-Hsing University, Institute of Biomedical Sciences, Taichung City, Taiwan.
- 1850 Jikei University School of Medicine, Research Center for Medical Sciences, Division of Gene Therapy, Tokyo, Japan.
- 1851 Tokyo Medical and Dental University, Department of Pathological Cell Biology, Tokyo, Japan.
- 1852 Tohoku University, Graduate School of Agricultural Science, Sendai, Japan.
- 1853 Geisel School of Medicine at Dartmouth, Department of Biochemistry and Cell Biology, Hanover, NH, USA.
- 1854 Kobe University Graduate School of Medicine, Center for Infectious Diseases, Division of Infectious Disease Control, Kobe, Japan.
- 1855 MACS - Agharkar Research Institute, Developmental Biology Group, Pune, India; Savitribai Phule Pune University, Ganeshkhind, Pune, Maharashtra, India.
- 1856 Mayo Clinic College of Medicine, Department of Experimental Pathology, Rochester, MN, USA.
- 1857 National Sun Yat-sen University, Institute of Biopharmaceutical Sciences, Kaohsiung, Taiwan.
- 1858 Huazhong University of Science and Technology, College of Life Science and Technology, Key Laboratory of Molecular Biophysics of Ministry of Education,Wuhan, Hubei, China.
- 1859 University of Calgary, Cumming School of Medicine, Departments of Medical Genetics and Biochemistry & Molecular Biology, Calgary, Alberta, Canada.
- 1860 Department of Experimental and Health Sciences, Pompeu Fabra University (UPF), CIBER on Neurodegenerative diseases (CIBERNED), E-08003 Barcelona, Spain.
- 1861 Achucarro Basque Center for Neuroscience, Department of Neurosciences, University of the Basque Country EHU/UPV, Leioa, Bizkaia, Spain.
- 1862 Universidad de la República, Facultad de Agronomía, Departamento de Biología Vegetal, Montevideo, Uruguay.
- 1863 National Institutes of Health, NIEHS, RTP, Durham, NC, USA.
- 1864 David Geffen School of Medicine at University of California, Los Angeles, Department of Medicine, Division of Dermatology, Los Angeles, CA, USA.
- 1865 University of Bern, Institute of Pharmacology, Bern, Switzerland; Sechenov University, Department of Clinical Immunology and Allergology, Moscow, Russia.
- 1866 INSERM UMR1163, Laboratory of Epithelial Biology and Disease, Imagine Institute, Paris Descartes University, Sorbonne Paris Cité, Hôpital Necker-Enfants Malades, Paris, France.
- 1867 Boston University School of Medicine, Division of Hematology & Medical Oncology, Department of Pharmacology & Experimental Therapeutics, Boston, MA, USA.
- 1868 Wayne State University School of Medicine, Department of Ophthalmology, Visual and Anatomical Sciences, Detroit, MI, USA.
- 1869 University of Pittsburgh, Department of Pharmacology & Chemical Biology, Pittsburgh, PA, USA.
- 1870 Post Graduate Institute of Medical Education and Research (PGIMER), Department of Urology, Chandigarh, India.
- 1871 Biomedical Research Institute of New Mexico, VA Healthcare System, Albuquerque, NM, USA.
- 1872 CSIR-National Physical Laboratory, Dr. K. S. Krishnan Marg, New Delhi, India.
- 1873 University of Pittsburgh School of Medicine, Departments of Ophthalmology, Cell Biology, and Developmental Biology, Pittsburgh, PA, USA.
- 1874 Sanjay Gandhi Postgraduate Institute of Medical Sciences, Department of Endocrinology, Lucknow, India.
- 1875 North Dakota State University, Department of Chemistry and Biochemistry, Fargo, ND, USA.
- 1876 National Jewish Health, Department of Medicine, Division of Allergy & Immunology, and Division of Pulmonary and Critical Care Medicine, Denver, CO, USA.
- 1877 Masaryk University, Faculty of Medicine, Department of Biology, Brno, Czech Republic.
- 1878 Washington State University, Institute of Biological Chemistry, Pullman, WA, USA.
- 1879 Edinburgh Cancer Research UK Centre, MRC Institute of Genetics and Molecular Medicine, University of Edinburgh, Edinburgh, UK.
- 1880 Dong-A University, College of Medicine, Department of Molecular Neuroscience, Peripheral Neuropathy Research Center, Busan, Korea.
- 1881 Lomonosov Moscow State University, Department of Biology, Moscow, Russia.
- 1882 Indian Institute of Technology Indore, Discipline of Biosciences and Biomedical Engineering, Indore, Madhya Pradesh, India.
- 1883 Shandong University, School of public health, Department of Toxicology and nutrition. Jinan, Shandong, China.
- 1884 Korea University, Department of Life Sciences, Seoul, Korea.
- 1885 Guangzhou University of Chinese Medicine, Medical College of Acupuncture-Moxibustion and Rehabilitation, Guangzhou, China; Hong Kong Baptist University, School of Chinese Medicine, Mr. and Mrs. Ko Chi Ming Centre for Parkinson's Disease Research, Hong Kong SAR, China.
- 1886 University of Colorado Anschutz Medical Campus, Division of Cardiology, Aurora, CO, USA.
- 1887 Hubei Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan, Hubei, China.
- 1888 Massachusetts General Hospital and Harvard Medical School, Department of Medicine, Endocrine Division, Boston, MA, USA.
- 1889 University of Guelph, Department of Food Science, Guelph, Canada.
- 1890 University of California, San Diego, La Jolla, CA, USA; Rady Children's Hospital San Diego, San Diego, CA, USA.
- 1891 Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bengaluru-560029, Karnataka, India.
- 1892 University of Kentucky, Department of Toxicology and Cancer Biology, Lexington, KY, USA.
- 1893 Institute of Molecular Biology and Pathology, National Research Council (CNR), Rome, Italy; Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione Santa Lucia, Laboratory of Cell Signaling, Rome, Italy.
- 1894 University of Western Ontario, Department of Physiology and Pharmacology, London, Ontario, Canada.
- 1895 University of Oslo, Department of Neuro-/Pathology, Oslo, Norway.
- 1896 University of Bonn, Department of Pharmaceutical & Cellbiological Chemistry, Bonn, Germany.
- 1897 Biomedical Research Foundation of the Academy of Athens, Laboratory of Neurodegenerative Diseases, Athens, Greece; National and Kapodistrian University of Athens Medical School, First Department of Neurology, Athens, Greece.
- 1898 University of California, Irvine, Department of Psychiatry and Human Behavior, Irvine, CA, USA.
- 1899 Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Immune Modulation, University Hospital Erlangen, Erlangen, Germany.
- 1900 Oslo University Hospital, Institute for Cancer Research, Department of Molecular Cell Biology, Montebello, Oslo, Norway.
- 1901 Technische Universität Dresden, Center for Regenerative Therapies Dresden, Dresden, Germany.
- 1902 Jožef Stefan Institute, Department of Biochemistry and Molecular and Structural Biology, Ljubljana, Slovenia.
- 1903 University Medical Center Hamburg-Eppendorf, Children's Hospital, University Children's Research@Kinder-UKE, Hamburg, Germany.
- 1904 Heinrich-Heine-University, Medical Faculty, Institute of Molecular Medicine I, Düsseldorf, Germany.
- 1905 IRCCS Fondazione Santa Lucia, Rome, Italy.
- 1906 The Ohio State University, Department of Cancer Biology and Genetics, Columbus, OH, USA.
- 1907 University of California San Diego, UCSD Moores Cancer Center, La Jolla, CA, USA.
- 1908 Peking Union Medical College and Chinese Academy of Medical Sciences, Peking Union Medical College Hospital, Department of Critical Care Medicine, Beijing, China.
- 1909 Instituto Oswaldo Cruz, LITEB/IOC, Fundação Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, Brazil.
- 1910 Case Western Reserve University School of Medicine, Departments of Medicine and Pathology, Cleveland, OH USA.
- 1911 Rutgers University, New Jersey Medical School and Public Health Research Institute, Newark, NJ, USA.
- 1912 University of Madras, Department of Biochemistry, Guindy Campus, Chennai, India.
- 1913 Kolling Institute, Department of Neurogenetics, University of Sydney and Royal North Shore Hospital, St Leonards NSW, Australia.
- 1914 Hangzhou Normal University, Affiliated Hospital of Hangzhou Normal University, College of Medicine, Holistic Integrative Pharmacy Institutes and Comprehensive Cancer Diagnosis and Treatment Center, Hangzhou, Zhejiang, China.
- 1915 Iowa State University, Department of Kinesiology, Ames, IA, USA.
- 1916 University of Nebraska Lincoln, Department of Agronomy and Horticulture, Center for Plant Science Innovation, Lincoln, NE, USA.
- 1917 University of Illinois at Chicago, Department of Medicine, Chicago, IL, USA.
- 1918 Shanghai Jiao Tong University, School of Medicine, Ren Ji Hospital, Center for Reproductive Medicine, Shanghai, China.
- 1919 Wuhan University, College of Life Sciences, Wuhan, Hubei, China.
- 1920 Zhejiang University, School of Medicine, Department of Biochemistry, Hangzhou, Zhejiang, China.
- 1921 Cancer Institute of the Second Affiliated Hospital and Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
- 1922 University of Tennessee Health Science Center, Department of Physiology, Memphis, TN, USA.
- 1923 Experimental Physiopathology, Department of Sciences/Experimental Physiopatholgy, Medical School, University of São Paulo, Brazil.
- 1924 Swedish University of Agricultural Sciences, The Linnean Centre of Plant Biology in Uppsala, Department of Plant Biology, Uppsala, Sweden.
- 1925 University of Pennsylvania, Perelman School of Medicine, Department of Medicine, Philadelphia, PA, USA.
- 1926 University of Missouri, Division of Animal Science and Department of Obstetrics, Gynecology & Women's Health, Columbia, MO, USA.
- 1927 Tokai University School of Medicine, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Isehara, Kanagawa, Japan.
- 1928 York University, Department of Biology, Toronto, Canada.
- 1929 University of Utah, Department of Nutrition and Integrative Physiology; Division of Endocrinology, Metabolism, and Diabetes; Investigator, Molecular Medicine Program, Salt Lake City, UT, USA.
- 1930 Hong Kong Baptist University, Faculty of Science, Department of Biology, Hong Kong, China; Hong Kong Baptist University, Golden Meditech Centre for NeuroRegeneration Sciences, Hong Kong, China.
- 1931 Ohio Musculoskeletal and Neurological Institute (OMNI) and Department of Biomedical Sciences, Heritage College of Osteopathic Medicine, Ohio University, Athens, OH, USA.
- 1932 Istituto Superiore di Sanità, Department of Oncology and Molecular Medicine, Rome, Italy; Fondazione Umberto Veronesi, Milan, Italy.
- 1933 Charité University Medicine Berlin, Department of Hepatology & Gastroenterology, Berlin, Germany.
- 1934 The University of Texas Health Science Center at Houston, McGovern Medical School, Department of Internal Medicine, Cardiology, Houston, TX, USA.
- 1935 Chengdu Medical College, Department of Anatomy and Histology and Embryology, Development and Regeneration Key Lab of Sichuan Province, Chengdu, China.
- 1936 University of Glasgow, Institute of Cancer Sciences, Glasgow, UK.
- 1937 Penn State University College of Medicine, Department of Pediatrics, Hershey, PA, USA.
- 1938 VIT University, School of Biosciences and Technology, Vellore, Tamilnadu, India.
- 1939 The Chinese University of Hong Kong, School of Biomedical Sciences, Hong Kong, China; The Chinese University of Hong Kong, Department of Obstetrics and Gynecology, Hong Kong, China.
- 1940 Inserm Unit 1195 - University of Paris Saclay, Le Kremlin-Bicetre, France.
- 1941 Tokyo Medical and Dental University (TMDU), Institute of Biomaterials and Bioengineering, Tokyo, Japan.
- 1942 National Neuroscience Institute, Duke NUS Medical School, Singapore.
- 1943 Key Laboratory of Oral Biomedicine Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, China.
- 1944 The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST), Wuhan University, School and Hospital of Stomatology, Department of Oral Medicine, Wuhan, Hubei, China.
- 1945 Kyoto Prefectural University of Medicine, Department of Anatomy and Neurobiology, Kyoto, Japan.
- 1946 RIKEN Center for Brain Science, Laboratory for Protein Conformation Diseases, Wako, Saitama, Japan.
- 1947 Hubei University of Technology, National "111" Center for Cellular Regulation and Molecular Pharmaceutics, Wuhan, China.
- 1948 State Key Laboratory of Membrane Biology, Institute of Zoology, Institute for Stem Cell and Regeneration, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Beijing, China.
- 1949 Juntendo University Graduate School of Medicine, Department of Cellular and Molecular Neuropathology, Tokyo, Japan.
- 1950 Harvard Medical School, Massachusetts General Hospital, Cutaneous Biology Research Center, Charlestown, MA, USA.
- 1951 University Medicine Goettingen, Neurology, Department of Translational Neurodegeneration, Center for Biostructural Imaging of Neurodegeneration (BIN), Goettingen, Germany.
- 1952 Foundation for Research and Technology-Hellas, Institute of Molecular Biology and Biotechnology, Heraklion, Crete, Greece; University of Crete, Medical School, Heraklion, Crete, Greece.
- 1953 Duke University, Department of Medicine; Duke University, Department of Molecular Genetics and Microbiology; Duke University, Department of Immunology, Durham, NC, USA; Geriatric Research, Education, and Clinical Center, VA Health Care Center, Durham, NC, USA.
- 1954 University of North Carolina, Department of Pathology, Chapel Hill, NC, USA.
- 1955 Nasonova Research Institute of Rheumatology, Moscow, Russia.
- 1956 Cardiff University, Division of Cancer and Genetics, Heath Park Way, Cardiff, UK.
- 1957 Goethe-University, Faculty of Medicine, Department of Clinical Pharmacology, Frankfurt, Germany.
- 1958 INSERM U955, Team "Virus Hepatology Cancers", Créteil, France; Université Paris-Est, UMR S955, UPEC, Créteil, France.
- 1959 Chulalongkorn University, Faculty of Allied Health Sciences, Age-Related Inflammation and Degeneration Research Unit, Department of Clinical Chemistry, Bangkok, Thailand.
- 1960 University of Liverpool, Department of Cellular and Molecular Physiology, Liverpool, UK.
- 1961 Centro de Investigaciones Biológicas Margarita Salas, CIB-CSIC, Madrid, Spain.
- 1962 Carl von Ossietzky University Oldenburg, School of Medicine and Health Sciences, Department for Neuroscience, Oldenburg, Germany.
- 1963 University of Genova, Department of Internal Medicine, Genova, Italy.
- 1964 University of Kansas Medical Center, Department of Otolaryngology, Kansas City, KS, USA.
- 1965 University of Nebraska Medical Center, Departments of Internal Medicine and Biochemistry and Molecular Biology, Omaha, NE, USA.
- 1966 University of Colorado School of Medicine, Department of Pharmacology, Aurora, CO, USA.
- 1967 CSIR-Institute of Genomics and Integrative Biology, South Campus, New Delhi, India.
- 1968 Hannover Medical School, Institute for Molecular and Therapeutic Strategies (IMTTS), Hannover, Germany.
- 1969 University Medical Center Göttingen, Institute of Cellular Biochemistry, Göttingen, Germany.
- 1970 South China Agricultural University, Guangdong Provincial Key Laboratory of Agro-animal Genomics and Molecular Breeding/Guangdong Provincial Sericulture and Mulberry Engineering Research Center, College of Animal Science, Guangzhou, China.
- 1971 University Hospital of Bonn, Institute of Reconstructive Neurobiology, Bonn, Germany.
- 1972 University of Antwerp, Department of Biomedical Sciences, Peripheral Neuropathy Research Group, Antwerp, Belgium.
- 1973 Concordia University, Department of Biology, Montreal, Quebec, Canada.
- 1974 Wayne State University School of Medicine, Department of Pharmacology, Detroit, MI, USA.
- 1975 University of Oviedo, Department of Functional Biology, Physiology, Oviedo, Asturias, Spain.
- 1976 Zhejiang University, Life Sciences Institute and Innovation Center for Cell Signaling Network, Hangzhou, China.
- 1977 Northwestern University, Feinberg School of Medicine, Department of Pathology, Chicago, IL, USA.
- 1978 Centre de Recherche des Cordeliers, INSERM, Université de Paris, Sorbonne Université, Paris, France.
- 1979 Nagoya University Graduate School of Medicine, Department of Pathology and Biological Responses, Nagoya, Japan.
- 1980 University of Belgrade, Faculty of Medicine, Institute of Microbiology and Immunology, Belgrade, Serbia.
- 1981 University Federico II of Naples, Department of Molecular Medicine and Medical Biotechnology, Naples, Italy.
- 1982 Universidade Federal do Rio de Janeiro, Institute of Biophysics Carlos Chagas Filho, Immunobiology Program, Rio de Janeiro, RJ, Brazil.
- 1983 National and Kapodistrian University of Athens, Faculty of Biology, Department of Cell Biology and Biophysics, Panepistimiopolis, Athens, Greece.
- 1984 Medical University of South Carolina, Department of Medicine, Division of Rheumatology & Immunology, Charleston, SC, USA.
- 1985 University of Bern, Institute of Pathology, Division of Experimental Pathology, Bern, Switzerland.
- 1986 University of Hong Kong, Cardiology Division, Department of Medicine, Hong Kong, China.
- 1987 University of Southampton , Biological Sciences, Faculty of Environmental and Life Sciences, Highfield, Southampton, UK.
- 1988 Universite de Moncton, Department of Chemistry and Biochemistry, Moncton, NB, Canada.
- 1989 University of Birmingham, Institute of Cancer and Genomic Sciences, Birmingham, UK.
- 1990 Weill Cornell Medicine, Department of Pathology and Laboratory Medicine, New York, NY, USA.
- 1991 Komarov Botanical Institute RAS, Laboratory of Molecular and Ecological Physiology, Saint Petersburg, Russian Federation.
- 1992 Nottingham Trent University, School of Science and Technology, Nottingham, UK.
- 1993 University of Oxford, John Radcliffe Hospital, Translational Gastroenterology Unit and Department of Paediatrics, Oxford, and Oxford NIHR Biomedical Research Centre, Oxford, UK.
- 1994 Institute of Psychiatry and Neurology, First Department of Neurology, Warsaw, Poland.
- 1995 Sechenov First Moscow State Medical University, Institute for Regenerative Medicine, Moscow, Russia.
- 1996 Mie University, Graduate School of Bioresources, Tsu, Japan.
- 1997 University of Osnabrück, Department of Biology/Chemistry, and Center of Cellular Nanoanalytics Osnabrück (CellNanOs), Osnabrück, Germany.
- 1998 Nagoya City University Graduate School of Medical Sciences, Department of Nephro-urology, Nagoya, Aichi, Japan.
- 1999 University of Tübingen, Center for Plant Molecular Biology (ZMBP), Tübingen, Germany.
- 2000 University of Milan, Department of Biosciences, Milan, Italy.
- 2001 University of Buenos Aires, Institute of Biochemistry and Molecular Medicine, Pathophysiology Department, School of Pharmacy and Biochemistry, CONICET, Buenos Aires, Argentina.
- 2002 University of Oxford, Nuffield Department of Clinical Neurosciences, Oxford, UK.
- 2003 Karolinska Institute, Department of Physiology and Pharmacology, Stockholm, Sweden.
- 2004 University of Murcia, Department of Biochemistry, Molecular Biology B and Immunology; Biomedical Research Institute of Murcia (IMIB-Arrixaca), Unit of Autophagy, Immunity and Cancer, Murcia, Spain.
- 2005 Fundación Instituto Leloir and IIBBA, CONICET, Buenos Aires, Argentina; Laboratorio de Glicobiología Celular y Genética Aplicada de Levaduras, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina.
- 2006 Instituto de Investigaciones Biomedicas Alberto Sols (CSIC-UAM), Madrid, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERdem), Instituto de Salud Carlos III, Madrid, Spain.
- 2007 Zhejiang University, School of Medicine, Department of Microbiology and Parasitology, Hangzhou, China.
- 2008 Vanderbilt University School of Medicine, Department of Pathology, Microbiology and Immunology, Nashville, TN, USA.
- 2009 Center of Translational Immunlogy, University Medical Center Utrecht, Utrecht, The Netherlands.
- 2010 Goethe University, Institute for Experimental Cancer Research in Pediatrics, Frankfurt am Main, Germany.
- 2011 University Hospitals Leuven, Department of Neurology, Leuven, Belgium; KU Leuven, Department of Neurosciences, Leuven, Belgium.
- 2012 Laboratory of Immunosenescence and Stem Cell Metabolism, Department of Oncology, Ludwig Cancer Institute, University of Lausanne, 1066 Epalinges, Switzerland.
- 2013 Scientific Institute, IRCCS E. Medea, Laboratory of Molecular Biology, Bosisio Parini, Lecco, Italy.
- 2014 Instituto de Investigaciones Biomedicas "Alberto Sols" (CSIC-UAM) and CIBERER (CIBER, ISCiii), Madrid, Spain.
- 2015 Harvard Medical School, Ophthalmology, Boston, MA, USA.
- 2016 Universitätsklinikum Essen, Institute for Cell Biology (Cancer Research), Essen, Germany.
- 2017 University of Groningen, University Medical Center Groningen, Department of Hematology, Groningen, The Netherlands.
- 2018 Tuscia University, Department of Ecological and Biological Sciences (DEB), Viterbo, Italy.
- 2019 EA 3842, Limoges University, Faculty of Medicine, Limoges cedex, France.
- 2020 Biomedical Research Foundation, Academy of Athens, Center of Clinical, Experimental Surgery and Translational Research, Athens, Greece.
- 2021 Institut de Pharmacologie et de Biologie Structurale, Université de Toulouse, CNRS, Université Paul Sabatier, Toulouse, France.
- 2022 University of Pittsburgh, Department of Pathology, Pittsburgh, PA, USA.
- 2023 Washington University in St. Louis, Department of Medicine, St. Louis, MO, USA.
- 2024 Service of Endocrinology, University Hospital Doctor Peset, Foundation for the Promotion of Health and Biomedical Research in the Valencian Region (FISABIO)-Department of Physiology, University of Valencia, Valencia, Spain.
- 2025 University of Montpellier, UMR 5235, Montpellier, France.
- 2026 Universidade NOVA de Lisboa, CEDOC, NOVA Medical School, Faculdade de Ciências Médicas, Lisboa, Portugal.
- 2027 University of Helsinki, Institute of Biotechnology, Electron Microscopy Unit, Helsinki, Finland.
- 2028 Vall d'Hebron Research Institute (VHIR)-Network Center for Biomedical Research in Neurodegenerative Diseases (CIBERNED)-Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain.
- 2029 Institute of Biomedicine of Valencia, Spanish Research Council (CSIC), Valencia, Spain.
- 2030 University Hospital La Paz Research Institute (IdiPAZ), Cancer and Human Molecular Genetics Area Oto-Neurosurgery Research Group, Madrid, Spain.
- 2031 University of Toulouse, Institute of Metabolic and cardiovascular Diseases, INSERM UMR 1048, Toulouse, France.
- 2032 Università Cattolica del Sacro Cuore, Department of Life Science and Public Health Section of Histology and Embryology, Rome, Italy.
- 2033 Italian Institute for Genomic Medicine (IIGM), Candiolo (TO), Italy; Candiolo Cancer Institute, FPO-IRCCS, Candiolo (TO), Italy.
- 2034 Simon Fraser University, Department of Chemistry, Department of Molecular Biology and Biochemistry, Burnaby, British Columbia, Canada.
- 2035 Institute for Systems Analysis and Computer Science "A. Ruberti", National Research Council (IASI-CNR), IRCCS Fondazione Santa Lucia, Rome, Italy.
- 2036 Eurac Research, Institute for Biomedicine, Bolzano, Italy.
- 2037 Rheinische Friedrich-Wilhelms-Universität, Institut für Biochemie und Molekularbiologie (IBMB), Bonn, Germany.
- 2038 University of Oxford, Oxford Parkinson's Disease Centre, Department of Physiology, Anatomy and Genetics, Oxford, UK.
- 2039 Fukushima Medical University, School of Medicine, Department of Anatomy and Histology, Fukushima, Japan.
- 2040 University of California, Los Angeles, Department of Integrative Biology and Physiology, Los Angeles, CA, USA.
- 2041 The University of Queensland, School of Chemistry and Molecular Biosciences and Australian Infectious Diseases Research Centre, St. Lucia, Brisbane, Queensland, Australia.
- 2042 Babraham Institute, Cambridge, UK.
- 2043 University of Bonn, Department of Neurology, Molecular Cell Biology Unit, Bonn, Germany.
- 2044 Universidad Autónoma de Madrid, Centro de Biología Molecular Severo Ochoa (CSIC-UAM & CIBERNED), Madrid, Spain.
- 2045 St Jude Children's Research Hospital, Department of Pathology, Memphis, TN, USA.
- 2046 Chang Gung University College of Medicine and Chang Gung Memorial Hospital, Department of Cardiology, Taoyuan City, Taiwan; National Health Research Institutes, Institute of Cellular and System Medicine, Zhunan, Taiwan.
- 2047 China Pharmaceutical University, School of Life Science and Technology, Nanjing, Jiangsu, China.
- 2048 University of California Los Angeles, School of Dentistry, Division of Oral Biology and Medicine, Los Angeles, CA, USA.
- 2049 Department of Bioinformatics, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China.
- 2050 University of Michigan, Department of Internal Medicine, Division of Hematology and Oncology, Ann Arbor, MI, USA.
- 2051 Shanghai Ninth People's Hospital, National Clinical Research Center for Oral Disease, Shanghai Jiao Tong Univeristy, School of Medicine, Shanghai, China.
- 2052 Texas A&M College of Dentistry, Department of Endodontics, Dallas, TX, USA.
- 2053 Third Military Medical University of China, Xinqiao Hospital, Institute of Respiratory Diseases, Chongqing, China.
- 2054 Soochow University, Center for Circadian Clocks; School of Biology & Basic Medical Sciences, Medical College, Suzhou, Jiangsu, China.
- 2055 CAS Center for Excellence in Nanoscience, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology (NCNST), Zhongguancun, Beijing, China.
- 2056 China Agricultural University, College of Biological Sciences, State Key Laboratory of Agro-Biotechnology and MOA Key Laboratory of Soil Microbiology, Beijing, China.
- 2057 Soochow University, Hematology Center of Cyrus Tang Hematology Institute, School of Medicine, Suzhou, China.
- 2058 China Academy of Chinese Medical Sciences, Artemisinin Research Center and the Institute of Chinese Materia Medica, Beijing, China.
- 2059 Shanxi Agricultural University, Shanxi Key Laboratory of Ecological Animal Science and Environmental Veterinary Medicine, Taigu, Shanxi, China.
- 2060 Sichuan University, West China School of Basic Medical Sciences & Forensic Medicine, Chengdu, China.
- 2061 Hunan Key Laboratory of Medical Epigenomics, Department of Dermatology, Second Xiangya Hospital, Central South University, Changsha, China.
- 2062 The Chinese University of Hong Kong, School of Public Health and Primary Care, Hong Kong, China.
- 2063 Fourth Military Medical University, College of Stomatology, Department of Oral Anatomy and Physiology and TMD, Xi'an, China.
- 2064 The First Affiliated Hospital, Jinan University, Guangzhou, China.
- 2065 Huazhong Agricultural University, College of Horticulture and Forestry Science, Key Laboratory of Horticultural Plant Biology (Ministry of Education), Wuhan, China.
- 2066 National University of Singapore, Department of Medicine, Cardiovascular Research Institute, Singapore.
- 2067 Feinstein Institutes for Medical Research, Center for Immunology and Inflammation, Manhasset, NY, USA.
- 2068 University of Kentucky, Department of Ophthalmology and Visual Sciences, Lexington, KY, USA.
- 2069 Zhujiang Hospital of Southern Medical University, Department of Neurology, Guangzhou, Guangdong Province, China.
- 2070 Cleveland Clinic, Department of Cardiovascular Medicine/Heart and Vascular Institute, Cleveland, OH, USA.
- 2071 Diabetes and Metabolism Research Institute, Department of Molecular & Cellular Endocrinology; City of Hope Medical Center, Beckman Research Institute, Comprehensive Cancer Center, Duarte, CA, USA.
- 2072 Xuzhou Medical University, Department of Anesthesiology, Xuzhou, Jiangsu, China.
- 2073 Stanford University School of Medicine, Department of Neurosurgery, Palo Alto, CA, USA.
- 2074 University of South Dakota, Division of Basic Biomedical Sciences, Vermillion, SD, USA.
- 2075 Department of Biomedical Sciences, College of Medicine, Florida State University, Tallahassee, FL, USA.
- 2076 Department of Neurology, University of Michigan School of Medicine, Ann Arbor, MI, USA.
- 2077 Kaohsiung Medical University, School of Dentistry, College of Dental Medicine, Kaohsiung, Taiwan.
- 2078 University of Southampton, Faculty of Environmental and Life Sciences, Biological Sciences, and the Institute for Life Sciences, Southampton, UK.
- 2079 German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany; Max-Planck-Institute for Metabolism Research, Hamburg Outstation, Hamburg, Germany; Shanghai Qiangrui Biotech Co., Ltd., Fengxian District, Shanghai, China.
- 2080 Nanjing Agricultural University, College of Horticulture, Weigang NO. 1, Nanjing, China.
- 2081 Saint Louis University, Department of Biology, Saint Louis, MO, USA.
- 2082 Air Force Medical University, Xijing Hospital and School of Basic Medicine, Department of Pathology, State Key Laboratory of Cancer Biology, Xi'an, China.
- 2083 Affiliated Union Hospital of Fujian Medical University, Fuzhou, Fujian, China.
- 2084 Wenzhou Medical University, Key Laboratory of Biotechnology and Pharmaceutical Engineering, School of Pharmaceutical Sciences, Wenzhou, China.
- 2085 Queen Mary University, Blizard Institute, Flow Cytometry Core Facility, London, UK.
- 2086 Juntendo University Graduate School of Medicine, Department of Metabolism & Endocrinology, Bunkyo-ku, Tokyo, Japan.
- 2087 Chiba University Graduate School of Medicine, Department of General Medical Science, Chiba, Japan.
- 2088 University of Cincinnati College of Medicine, Cincinnati Children's Research Foundation, Division of Pulmonary Biology, Department of Pediatrics, Cincinnati, OH, USA.
- 2089 Department of Biological Sciences, University of Denver, Denver, CO, USA.
- 2090 University of Pennsylvania, Department of Anesthesiology, Philadelphia, PA, USA.
- 2091 Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China.
- 2092 Washington University School of Medicine, Department of Neurology, St. Louis, MO, USA.
- 2093 University of Bonn, Pharmaceutical Institute, Section Pharmacology and Toxicology, Bonn, Germany.
- 2094 University Hospital RWTH Aachen, Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC), Aachen, Germany.
- 2095 Univ of Pittsburgh and Pittsburgh VA HealthSystem, Department of Pathology, Pittsburgh, PA, USA.
- 2096 Spencer Center for Vision Research, Byers Eye Institute, Stanford University School of Medicine, Stanford, CA, USA.
- 2097 Georg August University Göttingen, Institute of Microbiology and Genetics, Department of Genetics of Eukaryotic Microorganisms, Göttingen, Germany.
- 2098 Harvard Medical School and Brigham & Women's Hospital, Precision Neurology Program & APDA Center for Advanced Parkinson Research, Boston, MA, USA.
- 2099 University of Nottingham, School of Life Sciences, Queen's Medical Centre, Nottingham, UK.
- 2100 Scripps Research Institute, La Jolla, CA, USA.
- 2101 Amsterdam University Medical Centers, Location AMC, Tytgat Institute for Intestinal Research, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands.
- 2102 Quadram Institute Bioscience, Norwich Research Park, Norwich, Norfolk, UK.
- 2103 University of Edinburgh, MRC Institute of Genetics and Molecular Medicine, Scotland, UK.
- 2104 Heinrich-Heine-Universität Düsseldorf Institut für Physikalische Biologie, Düsseldorf, Germany.
- 2105 Queensland University of Technology, Centre for Tropical Crops and Biocommodities, Science and Engineering Faculty, Brisbane, Queensland, Australia.
- 2106 Royal Holloway University of London, Centre for Biomedical Sciences, Egham, Surrey, UK.
- 2107 National Institutes of Health, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
- 2108 University of Nebraska-Lincoln, Department of Plant Pathology, Lincoln, NE, USA.
- 2109 Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Department of Stem Cell Biology, Erlangen, Germany.
- 2110 University of Toronto, Faculty of Medicine, Department of Immunology, Toronto, ON, Canada.
- 2111 Department of Obstetrics and Gynecology, Weill Cornell Medicine, 1300 York Avenue, Box 35, New York, NY, United States.
- 2112 MFP CNRS UMR 5234, University of Bordeaux, Bordeaux, France.
- 2113 Institut Pasteur, Membrane Biochemistry and Transport, Paris, France.
- 2114 National University of Singapore, Yong Loo Lin School of Medicine, Healthy Longevity Translational Research Program, Singapore; National University Health System, Centre for Healthy Longevity, Singapore.
- 2115 School of Medicine, Shenzhen University, Shenzhen, China.
- 2116 Kanazawa University, WPI-Nano Life Science Institute (WPI-nanoLSI), Kanazawa, Ishikawa, Japan.
- 2117 University of Zurich, Institute of Experimental Immunology, Switzerland, Zurich.
- 2118 Southwest Medical University, Luzhou Key Laboratory of Activity Screening and Druggability Evaluation for Chinese Materia Medica, Jiangyang District, Luzhou, China.
- 2119 University of California San Diego, Department of Neurosciences, La Jolla, CA, USA.
- 2120 University of Maryland School of Medicine, Center for Shock, Trauma and Anesthesiology Research (STAR), Departments of Anesthesiology, Anatomy & Neurobiology, Baltimore, MD, USA.
- 2121 NHRI, Institute of Cellular and System Medicine, Zhunan, Taiwan.
- 2122 University of North Dakota, Biology School of Medicine and Health Sciences, Department of Biomedical Sciences, Grand Forks, ND, USA.
- 2123 Soochow University, Department of Pathogenic Biology, Suzhou, Jiangsu Province, China.
- 2124 First Affiliated Hospital of Xi'an Jiaotong University School of Medicine, Center for Translational Medicine, Xi'an, Shaanxi, China.
- 2125 Zhejiang Provincial People's Hospital, Department of Endocrinology, Hangzhou, China.
- 2126 University of Kansas, Department of Molecular Biosciences, and University of Kansas Medical School, University of Kansas Cancer Center, Department of Radiation Oncology, Lawrence, KS, USA.
- 2127 Zhejiang University School of Medicine, Department of Toxicology of School of Public Health, and Department of Gynecologic Oncology of Women's Hospital, Hangzhou,China.
- 2128 The Broad Institute of Harvard and MIT, Massachusetts General Hospital, Harvard Medical School, Department of Molecular Biology, Cambridge, MA, USA.
- 2129 Zhejiang University, Department of Biochemistry in School of Medicine, Hangzhou, Zhejiang Province, China.
- 2130 Shenzhen University, College of Medicine, Shenzhen, Guangdong, China.
- 2131 The University of Hong Kong, Department of Anesthesiology, Queen Mary Hospital, Hong Kong, China.
- 2132 Wuhan University, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education, School of Pharmaceutical Sciences, Wuhan, China.
- 2133 Shanghai Jiao Tong University School of Medicine, Ruijin Hospital, Department of Otolaryngology & Head and Neck Surgery, Shanghai, China.
- 2134 Friedrich-Alexander University Erlangen-Nürnberg, University Hospital Erlangen, Department of Molecular Neurology, Erlangen Germany.
- 2135 Research Department, National Neuroscience Institute, Singapore.
- 2136 Southern University of Science and Technology, Department of Biology, Shenzhen, China.
- 2137 Sichuan University, West China Hospital, Centre of Geriatrics and Gerontology, Chengdu, China.
- 2138 University of Electronic Science and Technology of China, School of Medicine, Chengdu, China; Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, Department of Pharmacy, Chengdu, Sichuan, China.
- 2139 Wenzhou Medical University, School of Pharmaceutical Sciences, Wenzhou, China.
- 2140 Queensland University of Technology, Institute of Health and Biomedical Innovation, Kelvin Grove, Brisbane, Queensland, Australia.
- 2141 Sun Yat-sen University, School of Life Sciences, Guangdong Provincial Key Laboratory of Plant Resources, State Key Laboratory of Biocontrol, Guangzhou, China.
- 2142 South China Agricultural University, College of Veterinary Medicine, Guangzhou, Guangdong, China.
- 2143 Southwest Hospital, Third Military Medical University (Army Medical University), Key Laboratory of Hepatobiliary and Pancreatic Surgery, Institute of Hepatobiliary Surgery, Chongqing, China.
- 2144 University of Alabama at Birmingham, Division of Cardiovascular Disease, Department of Medicine, Birmingham, AL, USA.
- 2145 Georgia State University, The Center for Molecular and Translation Medicine, Atlanta, GA, USA.
- 2146 Biomedical Research Foundation of the Academy of Athens (BRFAA), Center of Clinical, Experimental Surgery, & Translational Research, Athens, Greece.
- 2147 Shanghai Sixth People's Hospital, Department of Cardiovascular Medicine, and Shanghai Jiaotong University School of Medicine, Shanghai Institute of Immunology, Shanghai, China.
- 2148 Institute of Neurosciences and Department of Neurology of the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- 2149 Jinan University, College of Life Science and Technology, Guangzhou, China.
- 2150 Yangzhou University, College of Veterinary Medicine, Jiangsu Province, China.
- 2151 Kyoto Institute of Technology, Department of Applied Biology, Matsugasaki, Sakyo-ku, Kyoto, Japan.
- 2152 Ehime University Graduate School of Medicine, Department of Cardiology, Pulmonology, Hypertension & Nephrology, Shitsukawa, Toon, Ehime, Japan.
- 2153 Columbia University, Department of Neurology, and Pathology and Cell Biology, New York, NY, USA.
- 2154 Juntendo University School of Medicine, Department of Gastroenterology, Bunkyo-ku, Tokyo, Japan.
- 2155 Indiana University School of Medicine, Department of Pathology and Laboratory Medicine, Indianapolis, IN, USA.
- 2156 National Cheng Kung University, College of Medicine, Department of Physiology, Tainan City, Taiwan.
- 2157 University of Virginia, Departments of Medicine, Pharmacology, Molecular Physiology & Biological Physics, Center for Skeletal Muscle Research at Robert M. Berne Cardiovascular Research Center, University of Virginia School of Medicine, Charlottesville, VA, USA.
- 2158 Asahikawa Medical University, Department of Ophthalmology, Hokkaido, Japan.
- 2159 Hong Kong Baptist University, Mr. and Mrs. Ko Chi Ming Centre for Parkinson's Disease Research, School of Chinese Medicine, Hong Kong, China.
- 2160 Nathan Kline Institute for Psychiatric Research, Center for Dementia Research, Orangeburg, NY; New York University Langone Health, Department of Psychiatry, New York, NY, USA.
- 2161 University of Wisconsin-Madison, Department of Surgery, Madison, WI, USA.
- 2162 Chinese Academy of Sciences, Shanghai Institute of Nutrition and Health, CAS Key Laboratory of Tissue Microenvironment & Tumor, Laboratory of Molecular Cardiology, Shanghai, China.
- 2163 Capital Medical University, School of Basic Medical Sceinces, Department of Neurobiology, Beijing, China.
- 2164 University of Kentucky, Department of Cancer Biology & Toxicology, Lexington, KY, USA.
- 2165 University of Alabama at Birmingham, Department of Medicine, Division of Cardiovascular Disease, Birmingham, AL, USA.
- 2166 University of Iowa Carver College of Medicine, Fraternal Order of Eagles Diabetes Research Center, Department of Anatomy and Cell Biology, Iowa City, IA, USA.
- 2167 The Fourth Military Medical University, Xijing Hospital, Department of Orthopaedics, Xi'an, China.
- 2168 Shandong Cancer Hospital and Institute, Cancer Research Center, Jinan, Shandong Province, China.
- 2169 Taipei Medical University, College of Medical Science and Technology, Graduate Institute of Cancer Biology and Drug Discovery, Taipei, Taiwan.
- 2170 The Fourth Military Medical University, Tangdu Hospital, Department of Experimental Surgery, Xi'an, Shaanxi, China.
- 2171 Chung Shan Medical University, Institute of Medicine, Taichung, Taiwan.
- 2172 Jiangsu University, School of Medicine, Zhenjiang, Jiangsu, China.
- 2173 Yale University School of Medicine, Department of Comparative Medicine and Department of Cellular and Molecular Physiology, New Haven, CT, USA.
- 2174 Jinan University, Medical College, Key Laboratory for Regenerative Medicine of the Ministry of Education, Division of Histology and Embryology, Guangzhou, China.
- 2175 Hangzhou Normal University, School of Medicine, College of Pharmacy, Hangzhou, Zhejiang, China.
- 2176 University of Southeast, School of Medicine, Department of Pharmacology, Nanjing, JiangSu, China.
- 2177 University of Melbourne, Bio21 Molecular Science and Biotechnology Institute, Parkville, Victoria, Australia.
- 2178 Chinese University of Hong Kong, School of Biomedical Sciences, Hong Kong, China.
- 2179 Fourth Medical Center of the Chinese PLA General Hospital, Trauma Research Center, Beijing, China.
- 2180 Duke-NUS Medical School, Cardiovascular and Metabolic Disorders Program, Singapore, Singapore.
- 2181 Zhejiang University School of Medicine, Department of Biochemistry, and Department of Hepatobiliary and Pancreatic Surgery of the First Affiliated Hospital, Hangzhou, China.
- 2182 Shandong Agricultural University, College of Plant Protection, Shandong, China.
- 2183 Zhejiang University, College of Pharmaceutical Sciences, Hangzhou, China.
- 2184 Soochow University, College of Pharmaceutical Sciences, Department of Pharmacology, Suzhou, Jiangsu, China.
- 2185 The University of British Columbia, Department of Biochemistry and Molecular Biology, Vancouver, BC, Canada.
- 2186 Dong-A University College of Medicine, Department of Anatomy and Cell Biology, Busan, Korea.
- 2187 The Jikei University School of Medicine, Department of Biochemistry, Minato-ku, Tokyo, Japan.
- 2188 ETH Zürich, Institute of Biochemistry, Zürich, Switzerland.
- 2189 Tabriz University of Medical Sciences, Molecular Medicine Research Center, Tabriz, Iran.
- 2190 Hwa Chong Institution, Singapore, Singapore.
- 2191 Tianjin University of Traditional Chinese Medicine, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin, China.
- 2192 Temple University, Center for Metabolic Disease Research and Department of Physiology, Philadelphia, PA, USA.
- 2193 The Chinese University of Hong Kong, State Key Laboratory of Digestive Disease, Department of Medicine and Therapeutics, Hong Kong, China.
- 2194 Tsinghua University, School of Life Sciences, Tsinghua University-Peking University Joint Center for Life Sciences, State Key Laboratory of Membrane Biology, Beijing, China.
- 2195 Hepatobiliary Section, Department of Internal Medicine, Kaohsiung Medical University Hospital, and Hepatitis Research Center, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 2196 University of Toronto, Centre for Addiction and Mental Health, Toronto, Ontario, Canada.
- 2197 Air Force Medical University, Xijing Hospital, Department of Plastic and Reconstructive Surgery, Xi'an, China.
- 2198 Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 201203 Shanghai, China; Department of Cell Biology, Harvard Medical School, Boston, MA, USA.
- 2199 Central South University, The Second Xiang-Ya Hospital, National Clinical Research Center for Metabolic Diseases, Department of Metabolism and Endocrinology, Changsha, Hunan, China.
- 2200 Kaohsiung Medical University, Graduate Institute of Medicine, Kaohsiung, Taiwan.
- 2201 First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China.
- 2202 Beijing Institute of Basic Medical Sciences, Beijing, China.
- 2203 City University of Hong Kong, Department of Biomedical Sciences, Hong Kong, China.
- 2204 Icahn School of Medicine at Mount Sinai, Departments of Neurology and Neuroscience, New York, NY, USA.
- 2205 Graduate School of Dong-A University, College of Medicine, Department of Translational Biomedical Sciences, Busan, Korea.
- 2206 Centre for Genomic Regulation (CRG), Department of Cell and Developmental Biology, Barcelona, España.
- 2207 Laboratory of Pain and Signaling, Butantan Institute, Sao Paulo, SP, Brazil; Stanford University, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford, CA, USA.
- 2208 University of Brescia, Department of Molecular and Translational Medicine, Brescia, Italy.
- 2209 University of Texas Southwestern Medical Center, Department of Surgery, Dallas, TX, USA.
- 2210 Cancer Research UK Beatson Institute, Glasgow, UK; University of Glasgow, Institute of Cancer Sciences, Glasgow, UK.
- 2211 University of California, Davis, Department of Pathology and Laboratory Medicine, Shriners Hospitals for Children, Institute for Pediatric Regenerative Medicine, Sacramento, CA, USA.
- 2212 Tabriz University of Medical Sciences, Faculty of Advanced Medical Sciences, Department of Medical Nanotechnology, Tabriz, Iran.
- 2213 University Monastir, Faculty of Medicine, LR12ES05, Lab-NAFS 'Nutrition - Functional Food & Vascular Health', Monastir, Tunisia.
- 2214 University of Virginia, Department of Neuroscience, Charlottesville, VA, USA.
- 2215 Boston University, Department of Biomedical Engineering, Boston, MA, USA.
- 2216 University of Arizona, Department of Pharmacology and Toxicology, Tucson, AZ, USA.
- 2217 Peking University First Hospital, Renal Division, Xi Cheng District, Beijing, China.
- 2218 Chinese Academy of Sciences, Institute of Biophysics, National Laboratory of Biomacromolecules, Beijing, China.
- 2219 Zhejiang University School of Medicine, Department of Pathology, Hangzhou, China.
- 2220 University of Science & Technology of China, School of Life Sciences, Hefei, Anhui, China.
- 2221 Rutgers Robert Wood Johnson Medical School, Department of Neuroscience and Cell Biology, Piscataway, NJ, USA.
- 2222 Thomas Jefferson University/Vickie & Jack Farber Institute for Neuroscience, Hospital for Neuroscience, Philadelphia, PA, USA.
- 2223 Soochow University, Department of Pharmacology and Laboratory of Cerebrovascular Pharmacology, College of Pharmaceutical Science, Suzhou, Jiangsu, China.
- 2224 People's Hospital of Hangzhou Medical College, Zhejiang Provincial People's Hospital, Department of Oncology, Hangzhou, China; Zhejiang Provincial People's Hospital, Clinical Research Institute, Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
- 2225 University of Alabama at Birmingham, Department of Pathology, Birmingham, AL, USA.
- 2226 School of Radiation Medicine and Protection and State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, China.
- 2227 The Chinese University of Hong Kong, Department of Medicine and Therapeutics and Department of Anaesthesia and Intensive Care, Hong Kong, China.
- 2228 Huazhong Agricultural University, School of Veterinary Medicine/ Biomedical Center, Hongshan, Wuhan, China.
- 2229 Henan University of Technology, College of Bioengineering, Zhengzhou, China.
- 2230 University of Utah, Huntsman Cancer Institute and Department of Oncological Sciences, Salt Lake City, UT, USA.
- 2231 The University of Texas Health Science Center at Houston (UTHealth), McGovern Medical School, The Brown Foundation Institute of Molecular Medicine, Department of Neurobiology and Anatomy, Programs in Human and Molecular Genetics and Neuroscience of the MD Anderson UTHealth GSBS, Houston, TX, USA.
- 2232 Wake Forest Baptist Comprehensive Cancer Center, Department of Cancer Biology, Winston-Salem, NC, USA.
- 2233 Hungarian Academy of Sciences, Budapest, Hungary; Department of Anatomy, Cell and Developmental Biology, Eötvös Loránd University, Budapest, Hungary.
- 2234 The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Department of Orthopaedics, Wenzhou, Zhejiang Province, China.
- 2235 College of Respiratory and Critical Care Medicine, Chinese PLA General Hospital, Beijing, China.
- 2236 University of Newcastle, School of Biomedical Sciences and Pharmacy, NSW, Australia.
- 2237 University of Houston, College of Pharmacy, Department of Pharmacological & Pharmaceutical Sciences, Houston, TX, USA.
- 2238 University of Maryland, Molecular Virology Laboratory, VA-MD College of Veterinary Medicine, College Park, MD, USA.
- 2239 East China Normal University, Key Laboratory of Adolescent Health Assessment and Exercise Intervention of Ministry of Education, Shanghai, China.
- 2240 Nanjing Agricultural University, College of Plant Protection, Department of Plant Pathology, Nanjing, China.
- 2241 Department of Physiology, Department of Obstetrics/Gynecology, Wayne State University, Detroit, MI, USA.
- 2242 Rutgers University, Department of Surgery, New Brunswick, NJ, USA.
- 2243 Nanjing University of Chinese Medicine, Nanjing, China.
- 2244 Central China Normal University, College of Life Sciences, Wuhan, Hubei Province, China.
- 2245 The Fourth Military Medical University, Tangdu Hospital, Department of Neurosurgery, Xi'an, Shaanxi, China.
- 2246 Beijing Academy of Agriculture and Forestry Sciences, Institute of Plant and Environment Protection, Beijing, China.
- 2247 Shandong University, School of Life Sciences, Shandong Provincial Key Laboratory of Animal Cells and Developmental Biology, Qingdao, China.
- 2248 Peking University Health Sciences Center, Department of Biochemistry and Molecular Biology, Beijing, China.
- 2249 Zhejiang University, the First Affiliated Hospital, Hangzhou, Zhejiang, China.
- 2250 Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China.
- 2251 University of Sydney, Westmead Institute for Medical Research, Centre for Transplant and Renal Research, Sydney NSW, Australia.
- 2252 Shenzhen University, Health Science Center, School of Pharmaceutical Sciences, Shenzhen, Guangdong, China.
- 2253 Wuhan University, Department of Cell Biology, College of Life Sciences, Wuhan, Hubei, China.
- 2254 University of Calgary, Cumming School of Medicine, Libin Cardiovascular Institute of Alberta, Departments of Biochemistry & Molecular Biology and Physiology & Pharmacology, Calgary, Alberta, Canada.
- 2255 Children's Hospital Medical Center, Division of Experimental Hematology and Cancer Biology, Cincinnati, OH, USA.
- 2256 China Pharmaceutical University, State Key Laboratory of Natural Medicines, Nanjing, Jiangsu, China.
- 2257 Karolinska Institutet Division of Toxicology, Institute of Environmental Medicine, Stockholm, Sweden; Lomonosov Moscow State University, Faculty of Basic Medicine, Moscow, Russia.
- 2258 Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Department of Pathophysiology, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai, China.
- 2259 Southwest Medical University, Department of Medical Cellular Biology, Luzhou, Sichuan, China.
- 2260 Massachusetts General Hospital, Department of Medicine, Diabetes Unit and Center for Genomic Medicine, Boston, MA, USA; Harvard Medical School, Department of Medicine, Boston, MA, USA.
- 2261 Chinese People's Liberation Army General Hospital, Department of Cardiology, Beijing, China.
- 2262 Third Military Medical University, College of Pharmacy, Department of Pharmacology, Chongqing, China.
- 2263 Huazhong Agricultural University, College of Veterinary Medicine, State Key Laboratory of Agricultural Microbiology, Wuhan, China.
- 2264 Zhejiang University, Department of Horticulture, Hangzhou, China.
- 2265 Guangxi Medical University, School of Preclinical Medicine, Department of Physiology, Nanning,China.
- 2266 Zhejiang University, MOA Key Laboratory of Animal Virology, Hangzhou, Zhejiang, China.
- 2267 The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Department of Orthopaedics, Wenzhou, Zhejiang, China.
- 2268 Chinese Academy of Sciences, Guangzhou Institutes of Biomedicine and Health, CAS Key Laboratory of Regenerative Biology, Guangzhou, China.
- 2269 Queensland University of Technology, Institute of Health and Biomedical Innovation, Brisbane, QLD, Australia.
- 2270 Texas A&M University, Institute of Biosciences and Technology, Houston, TX, USA.
- 2271 Soochow University, Laboratory Animal Center, Suzhou, Jiangsu Province, China.
- 2272 Zhejiang University School of Medicine, Department of Environmental Medicine, Hangzhou, Zhejiang Province, China.
- 2273 University at Buffalo, The State University of New York, Department of Physiology and Biophysics, Buffalo, NY, USA.
- 2274 Zhengzhou University, Third Affiliated Hospital and Institute of Neuroscience, Henan Key Laboratory of Child Brain Injury, Zhengzhou, China; Gothenburg University, Institute of Neuroscience and Physiology, Gothenburg, Sweden.
- 2275 Nanjing Medical University, Department of Physiology, Nanjing, Jiangsu, China.
- 2276 University of Kentucky, College of Medicine, Department of Molecular and Cellular Biochemistry, Lexington, KY, USA.
- 2277 Shanghai Jiao Tong University, Bio-X Institutes, Shanghai, China.
- 2278 The Ohio State University, Department of Surgery, Columbus, OH, USA.
- 2279 Shenzhen University Medical School, Department of Biochemistry and Molecular Biology, Shenzhen, China.
- 2280 State Key Laboratory of Medicinal Chemical Biology, Tianjin Key Laboratory of Protein Science, College of Life Sciences, Nankai University, Tianjin, China.
- 2281 University California, San Diego, Division of Biological Sciences, Section of Molecular Biology, CA, USA.
- 2282 University Medical Center of Johannes Gutenberg-University, Institute for Virology, Mainz, Germany.
- 2283 University of Padova, Biology Department, Padova, Italy.
- 2284 Rutgers University, Department of Chemical Biology, Ernest Mario School of Pharmacy, Piscataway, NJ, USA.
- 2285 Moscow State University, A.N.Belozersky Institute of Physico-Chemical Biology, Department of Functional Biochemistry of Biopolymers, Moscow, Russia.
- 2286 University of Michigan, Departments of Surgery and Pathology, Ann Arbor, MI, USA.
- 2287 Institute of Life Sciences, Chongqing Medical University, Chongqing, PR China.
- 2288 The University of Queensland, Queensland Brain Institute, Clem Jones Centre for Ageing Dementia Research, Brisbane, Australia.
- 2289 University of Innsbruck, Division of Cell Metabolism and Differentiation Research, Research Institute for Biomedical Aging Research, Innsbruck, Austria.
- 2290 Indiana University School of Medicine, Department of Biochemistry and Molecular Biology, Indianapolis, IN, USA.
- 2291 Swedish University of Agricultural Sciences, Department of Forest Mycology and Plant Pathology, Uppsala, Sweden.
- 2292 Southern Medical University, Shenzhen Hospital, Department of Pathology, Bao'an District, Shenzhen, Guangdong, China.
- 2293 Lovelace Respiratory Research Institute, Molecular Biology and Lung Cancer Program, Albuquerque, NM, USA.
- 2294 Tokyo Medical and Dental University (TMDU), Medical Hospital, Department of Gastroenterology and Hepatology, Bunkyo-ku, Tokyo, Japan.
- 2295 Huazhong University of Science and Technology, School of Basic Medicine, Wuhan, HuBei, China.
- 2296 Maastricht University Medical Center, School for Cardiovascular disease (CARIM), Department of Pathology, Maastricht, The Netherlands; University of Edinburgh, Centre for Cardiovascular Science, Edinburgh, UK.
- 2297 Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO, USA.
- 2298 Hong Kong Baptist University, School of Chinese Medicine, Hong Kong, China.
- 2299 Hong Kong Baptist University, School of Chinese Medicine, Hong Kong, lxChina.
- PMID: 33634751
- PMCID: PMC7996087
- DOI: 10.1080/15548627.2020.1797280
Abstract
In 2008, we published the first set of guidelines for standardizing research in autophagy. Since then, this topic has received increasing attention, and many scientists have entered the field. Our knowledge base and relevant new technologies have also been expanding. Thus, it is important to formulate on a regular basis updated guidelines for monitoring autophagy in different organisms. Despite numerous reviews, there continues to be confusion regarding acceptable methods to evaluate autophagy, especially in multicellular eukaryotes. Here, we present a set of guidelines for investigators to select and interpret methods to examine autophagy and related processes, and for reviewers to provide realistic and reasonable critiques of reports that are focused on these processes. These guidelines are not meant to be a dogmatic set of rules, because the appropriateness of any assay largely depends on the question being asked and the system being used. Moreover, no individual assay is perfect for every situation, calling for the use of multiple techniques to properly monitor autophagy in each experimental setting. Finally, several core components of the autophagy machinery have been implicated in distinct autophagic processes (canonical and noncanonical autophagy), implying that genetic approaches to block autophagy should rely on targeting two or more autophagy-related genes that ideally participate in distinct steps of the pathway. Along similar lines, because multiple proteins involved in autophagy also regulate other cellular pathways including apoptosis, not all of them can be used as a specific marker for bona fide autophagic responses. Here, we critically discuss current methods of assessing autophagy and the information they can, or cannot, provide. Our ultimate goal is to encourage intellectual and technical innovation in the field.
Keywords: Autophagosome; LC3; cancer; flux; lysosome; macroautophagy; neurodegeneration; phagophore; stress; vacuole.
Conflict of interest statement
No potential conflict of interest was reported by the corresponding author.
Figures

Figure 19.
Assessing autophagy with multispectral imaging…
Figure 19.
Assessing autophagy with multispectral imaging cytometry. (A) Bright Detail Intensity (BDI) measures the…

Figure 20.
HiBiT-LC3B imitates endogenous LC3B response…
Figure 20.
HiBiT-LC3B imitates endogenous LC3B response upon autophagy perturbation. (A) Principle of the Autophagy…

Figure 21.
Regulation of the SQSTM1 protein…
Figure 21.
Regulation of the SQSTM1 protein during autophagy. (A) The level of SQSTM1 during…

Figure 22.
Figure 22.

Figure 23.
Confocal microscopy image of HCT116…
Figure 23.
Confocal microscopy image of HCT116 cells immunostained with antibody specific to human ATG12.…

Figure 24.
Automated WIPI1 puncta image acquisition…
Figure 24.
Automated WIPI1 puncta image acquisition and analysis monitors the induction and inhibition of…
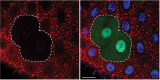
Figure 25.
Clonal analysis of autophagy in…
Figure 25.
Clonal analysis of autophagy in the Drosophila larval midgut. Inhibition of autophagy in…

Figure 26.
Figure 26.

Figure 27.
Human fibroblasts showing colocalization of…
Figure 27.
Human fibroblasts showing colocalization of mitochondria with lysosomes. The degree of colocalization of…

Figure 28.
Detection of mitophagy in primary…
Figure 28.
Detection of mitophagy in primary cortical neurons using mitochondria-targeted Keima. Neurons transfected with…

Figure 29.
PINK1-dependent phosphorylation of ubiquitin (p-S65-Ub)…
Figure 29.
PINK1-dependent phosphorylation of ubiquitin (p-S65-Ub) upon mitophagic stress. (A) Human dermal fibroblasts from…

Figure 30.
Confocal microscopy deconvolved (AutoQuant X3)…
Figure 30.
Confocal microscopy deconvolved (AutoQuant X3) images and colocalization image analysis (ImageJ 1.47; Imaris…

Figure 31.
Pathways that follow DNA damage…
Figure 31.
Pathways that follow DNA damage may result in PCD or cell survival through…

Figure 32.
LysoTracker™ Red stains lysosomes and…
Figure 32.
LysoTracker™ Red stains lysosomes and can be used to monitor autophagy in Drosophila.…

Figure 33.
GFP::LGG-1 and GFP::LGG-2 are autophagy…
Figure 33.
GFP::LGG-1 and GFP::LGG-2 are autophagy markers in C. elegans . (A-F) Animals were…

Figure 34.
Transmission electron micrograph of erythroblasts…
Figure 34.
Transmission electron micrograph of erythroblasts obtained from the blood of regular donors after…

Figure 35.
A large dystrophic neurite from…
Figure 35.
A large dystrophic neurite from a brain biopsy of a patient with Gerstmann-Sträussler-Scheinker…

Figure 36.
A high-power electron micrograph from…
Figure 36.
A high-power electron micrograph from a brain biopsy showing autophagic vacuoles in a…

Figure 37.
FIB-SEM images showing ultrastructural details…
Figure 37.
FIB-SEM images showing ultrastructural details of aging iPS cells. Arrows indicate autophagosomes containing…

Figure 38.
Autophagy in the digestive gland…
Figure 38.
Autophagy in the digestive gland of Ruditapes decussatus (Mollusca, Bivalvia) subjected to a…

Figure 39.
Detection of autophagy in Echinococcus…
Figure 39.
Detection of autophagy in Echinococcus granulosus larval stage. (A) Scanning electron micrographs of…

Figure 40.
Detection of autophagy in tobacco…
Figure 40.
Detection of autophagy in tobacco BY-2 cells. (A) Induction of autophagosomes in tobacco…

Figure 1.
Schematic model demonstrating the induction…
Figure 1.
Schematic model demonstrating the induction of autophagosome formation when turnover is blocked versus…

Figure 2.
An autophagic body in a…
Figure 2.
An autophagic body in a large lysosome of a mammalian epithelial cell in…
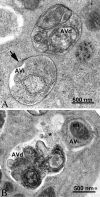
Figure 3.
TEM images of autophagic vacuoles…
Figure 3.
TEM images of autophagic vacuoles in isolated mouse hepatocytes. (A) One autophagosome or…

Figure 4.
Autophagosomes with recognizable cargo are…
Figure 4.
Autophagosomes with recognizable cargo are rare in cells. (A) To assess relative rates…

Figure 5.
Cryoelectron microscopy can be used…
Figure 5.
Cryoelectron microscopy can be used as a three-dimensional approach to monitor the autophagic…

Figure 6.
Different autophagic vacuoles observed after…
Figure 6.
Different autophagic vacuoles observed after freeze fracturing in cultured osteosarcoma cells after treatment…

Figure 7.
LC3-I conversion and LC3-II turnover.…
Figure 7.
LC3-I conversion and LC3-II turnover. (A) Expression levels of LC3-I and LC3-II during…

Figure 8.
Different LC3B-I:LC3B-II ratios indicating turnover…
Figure 8.
Different LC3B-I:LC3B-II ratios indicating turnover were assessed using a mono- as well as…

Figure 9.
Detection of nonlipidated (LC3-I) and…
Figure 9.
Detection of nonlipidated (LC3-I) and lipidated (LC3-II) forms of the LC3 protein using…

Figure 10.
Measuring autophagic flux and pool…
Figure 10.
Measuring autophagic flux and pool size of pathway intermediates at the single-cell level:…

Figure 11.
Effect of different inhibitors on…
Figure 11.
Effect of different inhibitors on LC3-II accumulation. SH-SY5Y human neuroblastoma cells were plated…

Figure 12.
GFP-LC3 processing can be used…
Figure 12.
GFP-LC3 processing can be used to monitor delivery of autophagosomal membranes. (A) atg5 …

Figure 13.
Movement of activated pDendra2-hp62 (SQSTM1;…
Figure 13.
Movement of activated pDendra2-hp62 (SQSTM1; orange) from the nucleus (middle) to an aggregate…

Figure 14.
The HaloTag-LC3 assay distinguishes phagophores,…
Figure 14.
The HaloTag-LC3 assay distinguishes phagophores, immature autophagosomes, and mature autophagosomes and autolysosomes. (A)…

Figure 15.
Changes in the detection and…
Figure 15.
Changes in the detection and localization of GFP-LC3 upon the induction of autophagy.…

Figure 16.
The GFP and mRFP signals…
Figure 16.
The GFP and mRFP signals of tandem fluorescent LC3 (tfLC3, mRFP-GFP-LC3) show different…

Figure 17.
GFP fluorescence in the autolysosome…
Figure 17.
GFP fluorescence in the autolysosome can be recovered upon neutralization of the pH.…
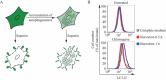
Figure 18.
Saponin extraction allows quantification of…
Figure 18.
Saponin extraction allows quantification of LC3-II fluorescence by FACS. (A) Schematic diagram of…












References
-
- Klionsky DJ, Cuervo AM, Seglen PO.. Methods for monitoring autophagy from yeast to human. Autophagy. 2007. May-Jun;3(3):181–206. PubMed PMID: 17224625; eng. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AR076092/AR/NIAMS NIH HHS/United States
- R01 DK015658/DK/NIDDK NIH HHS/United States
- MR/M00869X/2/MRC_/Medical Research Council/United Kingdom
- R01 AI083387/AI/NIAID NIH HHS/United States
- R01 DK108835/DK/NIDDK NIH HHS/United States
- K08 CA230151/CA/NCI NIH HHS/United States
- R01 CA211070/CA/NCI NIH HHS/United States
- R15 GM122035/GM/NIGMS NIH HHS/United States
- R35 GM127029/GM/NIGMS NIH HHS/United States
- R35 GM130331/GM/NIGMS NIH HHS/United States
- R01 DK114401/DK/NIDDK NIH HHS/United States
- BBS/E/B/000C0413/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 NS105971/NS/NINDS NIH HHS/United States
- R35 HL135736/HL/NHLBI NIH HHS/United States
- P01 CA140043/CA/NCI NIH HHS/United States
- MC_UU_00018/2/MRC_/Medical Research Council/United Kingdom
- R01 HL126933/HL/NHLBI NIH HHS/United States
- R01 ES029092/ES/NIEHS NIH HHS/United States
- K08 AI102971/AI/NIAID NIH HHS/United States
- 16337/CRUK_/Cancer Research UK/United Kingdom
- R01 HL088256/HL/NHLBI NIH HHS/United States
- R21 DE028256/DE/NIDCR NIH HHS/United States
- MR/S009426/1/MRC_/Medical Research Council/United Kingdom
- R01 DK057993/DK/NIDDK NIH HHS/United States
- R01 DK110162/DK/NIDDK NIH HHS/United States
- R01 AI130454/AI/NIAID NIH HHS/United States
- R01 CA140964/CA/NCI NIH HHS/United States
- R01 DK125513/DK/NIDDK NIH HHS/United States
- R01 CA229275/CA/NCI NIH HHS/United States
- R35 GM131681/GM/NIGMS NIH HHS/United States
- R35 GM131919/GM/NIGMS NIH HHS/United States
- R01 AR077440/AR/NIAMS NIH HHS/United States
- T32 DK007745/DK/NIDDK NIH HHS/United States
- R01 DK093668/DK/NIDDK NIH HHS/United States
- L30 AI091018/AI/NIAID NIH HHS/United States
- MR/S032304/1/MRC_/Medical Research Council/United Kingdom
- R01 AI087682/AI/NIAID NIH HHS/United States
- 15816/CRUK_/Cancer Research UK/United Kingdom
- T32 HL134621/HL/NHLBI NIH HHS/United States
- RF1 NS083704/NS/NINDS NIH HHS/United States
- R01 AG049780/AG/NIA NIH HHS/United States
- R01 AG061447/AG/NIA NIH HHS/United States
- R15 NS075684/NS/NINDS NIH HHS/United States
- R01 HL107594/HL/NHLBI NIH HHS/United States
- R01 CA252707/CA/NCI NIH HHS/United States
- I01 BX004444/BX/BLRD VA/United States
- R01 NS095634/NS/NINDS NIH HHS/United States
- P01 DK098108/DK/NIDDK NIH HHS/United States
- P30 ES006694/ES/NIEHS NIH HHS/United States
- R21 CA209345/CA/NCI NIH HHS/United States
- R01 NS103981/NS/NINDS NIH HHS/United States
- R01 AI136921/AI/NIAID NIH HHS/United States
- MR/N022696/1/MRC_/Medical Research Council/United Kingdom
- R35 CA232113/CA/NCI NIH HHS/United States
- R01 AG057509/AG/NIA NIH HHS/United States
- R01 HL133545/HL/NHLBI NIH HHS/United States
- MC_EX_G0800785/MRC_/Medical Research Council/United Kingdom
- K08 AG050808/AG/NIA NIH HHS/United States
- R01 GM118660/GM/NIGMS NIH HHS/United States
- R01 EY026885/EY/NEI NIH HHS/United States
- MR/R009732/1/MRC_/Medical Research Council/United Kingdom
- R01 DK124308/DK/NIDDK NIH HHS/United States
- R01 HL077328/HL/NHLBI NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- R15 GM102846/GM/NIGMS NIH HHS/United States
- R01 DK130879/DK/NIDDK NIH HHS/United States
- R01 NS115403/NS/NINDS NIH HHS/United States
- R01 DK112698/DK/NIDDK NIH HHS/United States
- P30 AG024824/AG/NIA NIH HHS/United States
- R01 GM115517/GM/NIGMS NIH HHS/United States
- R01 DK124901/DK/NIDDK NIH HHS/United States
- R01 DK123447/DK/NIDDK NIH HHS/United States
- I01 BX004306/BX/BLRD VA/United States
- R01 CA160417/CA/NCI NIH HHS/United States
- R01 CA184137/CA/NCI NIH HHS/United States
- R35 HL145241/HL/NHLBI NIH HHS/United States
- R01 EY018341/EY/NEI NIH HHS/United States
- R01 CA238457/CA/NCI NIH HHS/United States
- R01 NS042023/NS/NINDS NIH HHS/United States
- R01 EY019643/EY/NEI NIH HHS/United States
- R56 AG063820/AG/NIA NIH HHS/United States
- MR/R025096/1/MRC_/Medical Research Council/United Kingdom
- R01 AI139046/AI/NIAID NIH HHS/United States
- R35 GM136325/GM/NIGMS NIH HHS/United States
- 29576/CRUK_/Cancer Research UK/United Kingdom
- P30 DK089503/DK/NIDDK NIH HHS/United States
- R01 HL141759/HL/NHLBI NIH HHS/United States
- R01 HL085629/HL/NHLBI NIH HHS/United States
- R15 NS104857/NS/NINDS NIH HHS/United States
- R01 HL118558/HL/NHLBI NIH HHS/United States
- 29800/CRUK_/Cancer Research UK/United Kingdom
- R01 HL153614/HL/NHLBI NIH HHS/United States
- PG/17/14/32867/BHF_/British Heart Foundation/United Kingdom
- P20 GM121176/GM/NIGMS NIH HHS/United States
- R35 GM131689/GM/NIGMS NIH HHS/United States
- R01 ES031253/ES/NIEHS NIH HHS/United States
- R01 HL132318/HL/NHLBI NIH HHS/United States
- MR/T002220/1/MRC_/Medical Research Council/United Kingdom
- MC_U105170648/MRC_/Medical Research Council/United Kingdom
- I01 BX001516/BX/BLRD VA/United States
- R01 DK108921/DK/NIDDK NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- G0501003/MRC_/Medical Research Council/United Kingdom
- R37 AI042999/AI/NIAID NIH HHS/United States
- R01 HL154147/HL/NHLBI NIH HHS/United States
- R01 GM101972/GM/NIGMS NIH HHS/United States
- C0483/MRF_/MRF_/United Kingdom
- R01 EY015537/EY/NEI NIH HHS/United States
- R01 AI111935/AI/NIAID NIH HHS/United States
- R01 CA244144/CA/NCI NIH HHS/United States
- R01 AI113919/AI/NIAID NIH HHS/United States
- R01 NS094154/NS/NINDS NIH HHS/United States
- R01 AR070837/AR/NIAMS NIH HHS/United States
- R01 AG064892/AG/NIA NIH HHS/United States
- R01 GM119160/GM/NIGMS NIH HHS/United States
- R01 CA227838/CA/NCI NIH HHS/United States
- 24453/CRUK_/Cancer Research UK/United Kingdom
- R35 GM119571/GM/NIGMS NIH HHS/United States
- R01 CA247992/CA/NCI NIH HHS/United States
- R01 AA019730/AA/NIAAA NIH HHS/United States
- R01 GM102297/GM/NIGMS NIH HHS/United States
- R01 NS089737/NS/NINDS NIH HHS/United States
- R21 NS102780/NS/NINDS NIH HHS/United States
- R01 NS115876/NS/NINDS NIH HHS/United States
- R01 HL153599/HL/NHLBI NIH HHS/United States
- R01 EY027733/EY/NEI NIH HHS/United States
- R01 NS110943/NS/NINDS NIH HHS/United States
- R01 DK121545/DK/NIDDK NIH HHS/United States
- R01 CA181196/CA/NCI NIH HHS/United States
- R01 GM111295/GM/NIGMS NIH HHS/United States
- R01 AI122176/AI/NIAID NIH HHS/United States
- R01 NS093362/NS/NINDS NIH HHS/United States
- P30 EY001583/EY/NEI NIH HHS/United States
- R01 AG062475/AG/NIA NIH HHS/United States
- K12 HD101368/HD/NICHD NIH HHS/United States
- R01 DK117965/DK/NIDDK NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- R01 NS118146/NS/NINDS NIH HHS/United States
- R01 NS083704/NS/NINDS NIH HHS/United States
- R01 NS093843/NS/NINDS NIH HHS/United States
- P50 AA011999/AA/NIAAA NIH HHS/United States
- R01 HL165786/HL/NHLBI NIH HHS/United States
- R01 NS110716/NS/NINDS NIH HHS/United States
- R01 CA190370/CA/NCI NIH HHS/United States
- RF1 AG058476/AG/NIA NIH HHS/United States
- 206444/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- R01 CA237536/CA/NCI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 DK099558/DK/NIDDK NIH HHS/United States
- R25 NS070682/NS/NINDS NIH HHS/United States
- P30 DK084567/DK/NIDDK NIH HHS/United States
- 29754/CRUK_/Cancer Research UK/United Kingdom
- BBS/E/F/000PR10355/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- I01 BX004235/BX/BLRD VA/United States
- R01 AI072648/AI/NIAID NIH HHS/United States
- R35 GM122536/GM/NIGMS NIH HHS/United States
- R01 CA197398/CA/NCI NIH HHS/United States
- R01 DK113170/DK/NIDDK NIH HHS/United States
- R01 AI145997/AI/NIAID NIH HHS/United States
- R01 HL072166/HL/NHLBI NIH HHS/United States
- R01 DK024031/DK/NIDDK NIH HHS/United States
- R01 CA200310/CA/NCI NIH HHS/United States
- R01 GM127791/GM/NIGMS NIH HHS/United States
- R01 DK107220/DK/NIDDK NIH HHS/United States
- R21 AI144883/AI/NIAID NIH HHS/United States
- R01 CA233794/CA/NCI NIH HHS/United States
- P01 AG054407/AG/NIA NIH HHS/United States
- MC_U105184308/MRC_/Medical Research Council/United Kingdom
- UKDRI-2002/MRC_/Medical Research Council/United Kingdom
- I01 BX000774/BX/BLRD VA/United States
- R01 AG062375/AG/NIA NIH HHS/United States
- R01 AG067664/AG/NIA NIH HHS/United States
- R01 DK121759/DK/NIDDK NIH HHS/United States
- R01 AI132697/AI/NIAID NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- R41 CA271967/CA/NCI NIH HHS/United States
- I01 BX003803/BX/BLRD VA/United States
- R01 AI124121/AI/NIAID NIH HHS/United States
- R01 AI054476/AI/NIAID NIH HHS/United States
- R01 AR061370/AR/NIAMS NIH HHS/United States
- R01 HL142879/HL/NHLBI NIH HHS/United States
- R01 EY029675/EY/NEI NIH HHS/United States
- R00 GM117218/GM/NIGMS NIH HHS/United States
- R01 HL123340/HL/NHLBI NIH HHS/United States
