Insight into the preferential N-binding versus O-binding of nitrosoarenes to ferrous and ferric heme centers
- PMID: 33634802
- PMCID: PMC8061117
- DOI: 10.1039/d0dt03604h
Insight into the preferential N-binding versus O-binding of nitrosoarenes to ferrous and ferric heme centers
Abstract
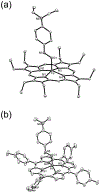
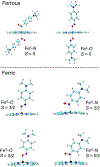
Nitrosoarenes (ArNOs) are toxic metabolic intermediates that bind to heme proteins to inhibit their functions. Although much of their biological functions involve coordination to the Fe centers of hemes, the factors that determine N-binding or O-binding of these ArNOs have not been determined. We utilize X-ray crystallography and density functional theory (DFT) analyses of new representative ferrous and ferric ArNO compounds to provide the first theoretical insight into preferential N-binding versus O-binding of ArNOs to hemes. Our X-ray structural results favored N-binding of ArNO to ferrous heme centers, and O-binding to ferric hemes. Results of the DFT calculations rationalize this preferential binding on the basis of the energies of associated spin-states, and reveal that the dominant stabilization forces in the observed ferrous N-coordination and ferric O-coordination are dπ-pπ* and dσ-pπ*, respectively. Our results provide, for the first time, an explanation why in situ oxidation of the ferrous-ArNO compound to its ferric state results in the observed subsequent dissociation of the ligand.
Conflict of interest statement
Conflicts of interest
“There are no conflicts to declare”.
Figures
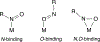





References
-
- Kiese M, Arch. Exptl. Pathol. Pharmakol, 1959, 235, 360–364.. - PubMed
-
- Jung F, Biochem. Z, 1940, 305, 248–260. Chem. Abs. 235:472.
-
- Belisario MA, Pecce R, Garofalo A, Sannolo N and Malorni A, Toxicology, 1996, 108, 101–108. - PubMed
-
- Harrison JH and Jollow DJ, J. Pharmacol. Exp. Ther, 1986, 238, 1045–1054. - PubMed
-
- Roldan M, Perez-Reinado E, Castillo F and Moreno-Vivian C, FEMS Microbiol. Rev, 2008, 32, 474–500. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

