In Silico Prediction of the Toxic Potential of Neuroprotective Bifunctional Molecules Based on Chiral N-Propargyl-1,2-amino Alcohol Derivatives
- PMID: 33635058
- PMCID: PMC8478334
- DOI: 10.1021/acs.chemrestox.0c00519
In Silico Prediction of the Toxic Potential of Neuroprotective Bifunctional Molecules Based on Chiral N-Propargyl-1,2-amino Alcohol Derivatives
Abstract
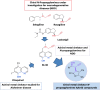
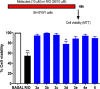
N-Propargylamines are useful synthetic scaffolds for the synthesis of bioactive molecules, and in addition, they possess important pharmacological activities. We obtained several neuroprotective molecules, chiral 1,2-amino alcohols and 1,2-diamines, able to reduce by almost 70% the rotenone and oligomycin A-induced damage in SH-SY5Y cells. Furthermore, some molecules assessed also counteracted the toxicity evoked by the Ser/Thr phosphatase inhibitor okadaic acid. Before extrapolating these data to preclinical studies, we analyze the molecules through an in silico prediction system to detect carcinogenicity risk or other toxic effects. In light of these promising results, these molecules may be considered as a lead family of neuroprotective and relatively safe compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents.Molecules. 2019 Oct 9;24(20):3647. doi: 10.3390/molecules24203647. Molecules. 2019. PMID: 31601055 Free PMC article.
-
Design, synthesis and evaluation of pterostilbene β-amino alcohol derivatives as multifunctional agents for Alzheimer's disease treatment.Bioorg Chem. 2018 Aug;78:298-306. doi: 10.1016/j.bioorg.2018.03.016. Epub 2018 Mar 28. Bioorg Chem. 2018. PMID: 29625269
-
Multipotent drugs with cholinergic and neuroprotective properties for the treatment of Alzheimer and neuronal vascular diseases. I. Synthesis, biological assessment, and molecular modeling of simple and readily available 2-aminopyridine-, and 2-chloropyridine-3,5-dicarbonitriles.Bioorg Med Chem. 2010 Aug 15;18(16):5861-72. doi: 10.1016/j.bmc.2010.06.095. Epub 2010 Jul 3. Bioorg Med Chem. 2010. PMID: 20656495
-
ITH12410/SC058: a new neuroprotective compound with potential in the treatment of Alzheimer's disease.ACS Chem Neurosci. 2014 Sep 17;5(9):770-5. doi: 10.1021/cn500131t. Epub 2014 Jul 15. ACS Chem Neurosci. 2014. PMID: 25008046 Review.
-
Synthesis of single-enantiomer bioactive molecules: a brief overview.Chirality. 2014 Feb;26(2):63-78. doi: 10.1002/chir.22268. Epub 2013 Dec 15. Chirality. 2014. PMID: 24339171 Review.
References
-
- Fast W.; Levsky M. E.; Marletta M. A.; Silverman R. B. (1997) N omega-propargyl-L-arginine and N omega-hydroxy-N omega-propargyl-L-arginine are inhibitors, but not inactivators, of neuronal and macrophage nitric oxide synthases. Bioorg. Med. Chem. 5 (8), 1601–1608. 10.1016/S0968-0896(97)00109-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

