Enantioselective α-Arylation of Ketones via a Novel Cu(I)-Bis(phosphine) Dioxide Catalytic System
- PMID: 33635068
- PMCID: PMC8041290
- DOI: 10.1021/jacs.0c13236
Enantioselective α-Arylation of Ketones via a Novel Cu(I)-Bis(phosphine) Dioxide Catalytic System
Abstract
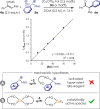
A novel catalytic system based on copper(I) and chiral bis(phosphine) dioxides is described. This allows the arylation of silyl enol ethers to access enolizable α-arylated ketones in good yields and enantiomeric excess up to 95%. Noncyclic ketones are amenable substrates with this method, which complements other approaches based on palladium catalysis. Optimization of the ligand structure is accomplished via rational design driven by correlation analysis. Preliminary mechanistic hypotheses are also evaluated in order to identify the role of chiral bis(phosphine) dioxides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Palucki M.; Buchwald S. L. Palladium-Catalyzed α-Arylation of Ketones. J. Am. Chem. Soc. 1997, 119, 11108. 10.1021/ja972593s. - DOI
-
- Hamann B. C.; Hartwig J. F. Palladium-Catalyzed Direct α-Arylation of Ketones. Rate Acceleration by Sterically Hindered Chelating Ligands and Reductive Elimination from a Transition Metal Enolate Complex. J. Am. Chem. Soc. 1997, 119, 12382. 10.1021/ja9727880. - DOI
-
- Hao Y.-J.; Hu X.-S.; Zhou Y.; Zhou J.; Yu J.-S. Catalytic Enantioselective α-Arylation of Carbonyl Enolates and Related Compounds. ACS Catal. 2020, 10, 955. 10.1021/acscatal.9b04480. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

