Expert Opinions on the Most Promising Treatments and Vaccine Candidates for COVID-19: Global Cross-sectional Survey of Virus Researchers in the Early Months of the Pandemic
- PMID: 33635275
- PMCID: PMC7919842
- DOI: 10.2196/22483
Expert Opinions on the Most Promising Treatments and Vaccine Candidates for COVID-19: Global Cross-sectional Survey of Virus Researchers in the Early Months of the Pandemic
Abstract
Background: The COVID-19 pandemic presents a great public health challenge worldwide, especially given the urgent need to identify effective drugs and develop a vaccine in a short period of time. Globally, several drugs and vaccine candidates are in clinical trials. However, because these drugs and vaccines are still being tested, there is still no definition of which ones will succeed.
Objective: This study aimed to assess the opinions of over 1000 virus researchers with knowledge on the prevention and treatment of coronavirus-related human diseases to determine the most promising drug and vaccine candidates to address COVID-19.
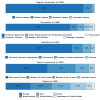
Methods: We mapped the clinical trials related to COVID-19 registered at ClinicalTrials.gov. These data were used to prepare a survey questionnaire about treatments and vaccine candidates for COVID-19. In May 2020, a global survey was conducted with authors of recent scientific publications indexed in the Web of Science Core Collection related to viruses, severe acute respiratory syndrome coronavirus, coronaviruses, and COVID-19.
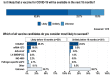
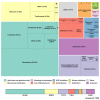
Results: Remdesivir, immunoglobulin from cured patients, and plasma were considered to be the most promising treatments in May 2020, while ChAdOx1 and mRNA-1273 were considered to be the most promising vaccine candidates. Almost two-thirds of the respondents (766/1219, 62.8%) believed that vaccines for COVID-19 were likely to be available in the next 18 months. Slightly fewer than 25% (289/1219, 23.7%) believed that a vaccine was feasible, but probably not within 18 months.
Conclusions: The issues addressed in this study are constantly evolving; therefore, the current state of knowledge has changed since the survey was conducted. However, for several months after the survey, the respondents' expectations were in line with recent results related to treatments and vaccine candidates for COVID-19.
Keywords: COVID-19; SARS-CoV-2; clinical trial; drug; public health; survey; treatment; vaccine.
©Bernardo Pereira Cabral, Luiza Braga, Fabio Mota. Originally published in JMIR Public Health and Surveillance (http://publichealth.jmir.org), 26.02.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
A Cross-sectional Survey of Public Knowledge and Perspective on Coronavirus Disease, Vaccination, and Related Research in India during the COVID-19 Pandemic.J Assoc Physicians India. 2023 Sep;71(9):19-27. doi: 10.59556/japi.71.0335. J Assoc Physicians India. 2023. PMID: 38700297
-
COVID-19: Underpinning Research for Detection, Therapeutics, and Vaccines Development.Pharm Nanotechnol. 2020;8(4):323-353. doi: 10.2174/2211738508999200817163335. Pharm Nanotechnol. 2020. PMID: 32811406 Review.
-
Consensus summary report for CEPI/BC March 12-13, 2020 meeting: Assessment of risk of disease enhancement with COVID-19 vaccines.Vaccine. 2020 Jun 26;38(31):4783-4791. doi: 10.1016/j.vaccine.2020.05.064. Epub 2020 May 25. Vaccine. 2020. PMID: 32507409 Free PMC article.
-
Primed for global coronavirus pandemic: Emerging research and clinical outcome.Eur J Med Chem. 2021 Jan 1;209:112862. doi: 10.1016/j.ejmech.2020.112862. Epub 2020 Sep 19. Eur J Med Chem. 2021. PMID: 33070079 Free PMC article. Review.
-
The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem.J Biol Regul Homeost Agents. 2021 Jan-Feb;35(1):1-4. doi: 10.23812/21-3-E. J Biol Regul Homeost Agents. 2021. PMID: 33377359
Cited by
-
Anticipating New Treatments for Cystic Fibrosis: A Global Survey of Researchers.J Clin Med. 2022 Feb 26;11(5):1283. doi: 10.3390/jcm11051283. J Clin Med. 2022. PMID: 35268374 Free PMC article.
References
-
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19) J Gen Intern Med. 2020 May 4;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. http://europepmc.org/abstract/MED/32133578 - DOI - PMC - PubMed
-
- WHO timeline - COVID-19. World Health Organization. [2020-05-23]. https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19.
-
- Coronavirus disease (COVID-19) pandemic. World Health Organization. [2020-05-23]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. World Health Organization. 2020. Mar 13, [2021-02-19]. https://www.who.int/docs/default-source/coronaviruse/clinical-management....
-
- COVID 19 landscape of experimental treatments. World Health Organization. [2021-02-19]. https://www.who.int/publications/i/item/covid-19-landscape-of-experiment....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

