Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility
- PMID: 33635866
- PMCID: PMC7909641
- DOI: 10.1371/journal.pgen.1009306
Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility
Abstract
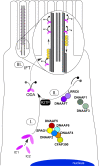
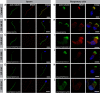
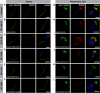
Axonemal protein complexes, such as outer (ODA) and inner (IDA) dynein arms, are responsible for the generation and regulation of flagellar and ciliary beating. Studies in various ciliated model organisms have shown that axonemal dynein arms are first assembled in the cell cytoplasm and then delivered into axonemes during ciliogenesis. In humans, mutations in genes encoding for factors involved in this process cause structural and functional defects of motile cilia in various organs such as the airways and result in the hereditary disorder primary ciliary dyskinesia (PCD). Despite extensive knowledge about the cytoplasmic assembly of axonemal dynein arms in respiratory cilia, this process is still poorly understood in sperm flagella. To better define its clinical relevance on sperm structure and function, and thus male fertility, further investigations are required. Here we report the fertility status in different axonemal dynein preassembly mutant males (DNAAF2/ KTU, DNAAF4/ DYX1C1, DNAAF6/ PIH1D3, DNAAF7/ZMYND10, CFAP300/C11orf70 and LRRC6). Besides andrological examinations, we functionally and structurally analyzed sperm flagella of affected individuals by high-speed video- and transmission electron microscopy as well as systematically compared the composition of dynein arms in sperm flagella and respiratory cilia by immunofluorescence microscopy. Furthermore, we analyzed the flagellar length in dynein preassembly mutant sperm. We found that the process of axonemal dynein preassembly is also critical in sperm, by identifying defects of ODAs and IDAs in dysmotile sperm of these individuals. Interestingly, these mutant sperm consistently show a complete loss of ODAs, while some respiratory cilia from the same individual can retain ODAs in the proximal ciliary compartment. This agrees with reports of solely one distinct ODA type in sperm, compared to two different ODA types in proximal and distal respiratory ciliary axonemes. Consistent with observations in model organisms, we also determined a significant reduction of sperm flagellar length in these individuals. These findings are relevant to subsequent studies on the function and composition of sperm flagella in PCD patients and non-syndromic infertile males. Our study contributes to a better understanding of the fertility status in PCD-affected males and should help guide genetic and andrological counselling for affected males and their families.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Tüttelmann F, Ruckert C, Röpke A. Spermatogenesestörungen: Perspektiven für erweiterte genetische Diagnostik nach 20 Jahren unveränderter Routine. Medizinische Genet. 2018;30: 12–20.
-
- WHO | Infertility is a global public health issue. WHO. 2019 [cited 4 Oct 2019]. Available from: https://www.who.int/reproductivehealth/topics/infertility/perspective/en/.
-
- Kliesch S. Diagnosis of male infertility: Diagnostic work-up of the infertile man. European Urology, Supplements. 2014;13(4): 73–82.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

