Transmission of SARS-CoV-2 in domestic cats imposes a narrow bottleneck
- PMID: 33635912
- PMCID: PMC7946358
- DOI: 10.1371/journal.ppat.1009373
Transmission of SARS-CoV-2 in domestic cats imposes a narrow bottleneck
Abstract
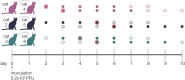
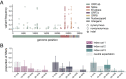
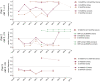
The evolutionary mechanisms by which SARS-CoV-2 viruses adapt to mammalian hosts and, potentially, undergo antigenic evolution depend on the ways genetic variation is generated and selected within and between individual hosts. Using domestic cats as a model, we show that SARS-CoV-2 consensus sequences remain largely unchanged over time within hosts, while dynamic sub-consensus diversity reveals processes of genetic drift and weak purifying selection. We further identify a notable variant at amino acid position 655 in Spike (H655Y), which was previously shown to confer escape from human monoclonal antibodies. This variant arises rapidly and persists at intermediate frequencies in index cats. It also becomes fixed following transmission in two of three pairs. These dynamics suggest this site may be under positive selection in this system and illustrate how a variant can quickly arise and become fixed in parallel across multiple transmission pairs. Transmission of SARS-CoV-2 in cats involved a narrow bottleneck, with new infections founded by fewer than ten viruses. In RNA virus evolution, stochastic processes like narrow transmission bottlenecks and genetic drift typically act to constrain the overall pace of adaptive evolution. Our data suggest that here, positive selection in index cats followed by a narrow transmission bottleneck may have instead accelerated the fixation of S H655Y, a potentially beneficial SARS-CoV-2 variant. Overall, our study suggests species- and context-specific adaptations are likely to continue to emerge. This underscores the importance of continued genomic surveillance for new SARS-CoV-2 variants as well as heightened scrutiny for signatures of SARS-CoV-2 positive selection in humans and mammalian model systems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
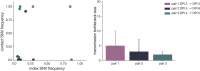
Update of
-
Transmission of SARS-CoV-2 in domestic cats imposes a narrow bottleneck.bioRxiv [Preprint]. 2021 Jan 4:2020.11.16.384917. doi: 10.1101/2020.11.16.384917. bioRxiv. 2021. Update in: PLoS Pathog. 2021 Feb 26;17(2):e1009373. doi: 10.1371/journal.ppat.1009373. PMID: 33236011 Free PMC article. Updated. Preprint.
Similar articles
-
Transmission of SARS-CoV-2 in domestic cats imposes a narrow bottleneck.bioRxiv [Preprint]. 2021 Jan 4:2020.11.16.384917. doi: 10.1101/2020.11.16.384917. bioRxiv. 2021. Update in: PLoS Pathog. 2021 Feb 26;17(2):e1009373. doi: 10.1371/journal.ppat.1009373. PMID: 33236011 Free PMC article. Updated. Preprint.
-
SARS-CoV-2 infection in domestic and feral cats: current evidence and implications.Vet Q. 2021 Dec;41(1):228-231. doi: 10.1080/01652176.2021.1962576. Vet Q. 2021. PMID: 34319851 Free PMC article.
-
Household Cases Suggest That Cats Belonging to Owners with COVID-19 Have a Limited Role in Virus Transmission.Viruses. 2021 Apr 14;13(4):673. doi: 10.3390/v13040673. Viruses. 2021. PMID: 33919936 Free PMC article.
-
Bats, pangolins, minks and other animals - villains or victims of SARS-CoV-2?Vet Res Commun. 2021 Feb;45(1):1-19. doi: 10.1007/s11259-021-09787-2. Epub 2021 Jan 19. Vet Res Commun. 2021. PMID: 33464439 Free PMC article. Review.
-
Viral phylodynamics.PLoS Comput Biol. 2013;9(3):e1002947. doi: 10.1371/journal.pcbi.1002947. Epub 2013 Mar 21. PLoS Comput Biol. 2013. PMID: 23555203 Free PMC article. Review.
Cited by
-
Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in Intercollegiate Athletics Not Fully Mitigated With Daily Antigen Testing.Clin Infect Dis. 2021 Jul 15;73(Suppl 1):S45-S53. doi: 10.1093/cid/ciab343. Clin Infect Dis. 2021. PMID: 33977295 Free PMC article.
-
SARS-COV-2 SEROSURVEY OF HEALTHY, PRIVATELY OWNED CATS PRESENTING TO A NEW YORK CITY ANIMAL HOSPITAL IN THE EARLY PHASE OF THE COVID-19 PANDEMIC (2020-2021).bioRxiv [Preprint]. 2024 Sep 3:2024.02.13.580068. doi: 10.1101/2024.02.13.580068. bioRxiv. 2024. PMID: 38405835 Free PMC article. Preprint.
-
Determinants and Mechanisms of the Low Fusogenicity and High Dependence on Endosomal Entry of Omicron Subvariants.mBio. 2023 Feb 28;14(1):e0317622. doi: 10.1128/mbio.03176-22. Epub 2023 Jan 10. mBio. 2023. PMID: 36625591 Free PMC article.
-
Intra- vs. Interhost Evolution of SARS-CoV-2 Driven by Uncorrelated Selection-The Evolution Thwarted.Mol Biol Evol. 2023 Sep 1;40(9):msad204. doi: 10.1093/molbev/msad204. Mol Biol Evol. 2023. PMID: 37707487 Free PMC article.
-
Experimental co-infection of calves with SARS-CoV-2 Delta and Omicron variants of concern.Emerg Microbes Infect. 2024 Dec;13(1):2281356. doi: 10.1080/22221751.2023.2281356. Epub 2023 Dec 30. Emerg Microbes Infect. 2024. PMID: 37938158 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

